Laser-scanning astrocyte mapping reveals increased glutamate-responsive domain size and disrupted maturation of glutamate uptake following neonatal cortical freeze-lesion
- PMID: 25249939
- PMCID: PMC4158796
- DOI: 10.3389/fncel.2014.00277
Laser-scanning astrocyte mapping reveals increased glutamate-responsive domain size and disrupted maturation of glutamate uptake following neonatal cortical freeze-lesion
Abstract
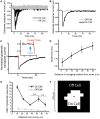
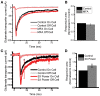
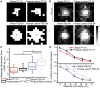
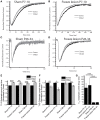
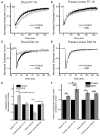
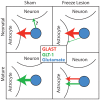
Astrocytic uptake of glutamate shapes extracellular neurotransmitter dynamics, receptor activation, and synaptogenesis. During development, glutamate transport becomes more robust. How neonatal brain insult affects the functional maturation of glutamate transport remains unanswered. Neonatal brain insult can lead to developmental delays, cognitive losses, and epilepsy; the disruption of glutamate transport is known to cause changes in synaptogenesis, receptor activation, and seizure. Using the neonatal freeze-lesion (FL) model, we have investigated how insult affects the maturation of astrocytic glutamate transport. As lesioning occurs on the day of birth, a time when astrocytes are still functionally immature, this model is ideal for identifying changes in astrocyte maturation following insult. Reactive astrocytosis, astrocyte proliferation, and in vitro hyperexcitability are known to occur in this model. To probe astrocyte glutamate transport with better spatial precision we have developed a novel technique, Laser Scanning Astrocyte Mapping (LSAM), which combines glutamate transport current (TC) recording from astrocytes with laser scanning glutamate photolysis. LSAM allows us to identify the area from which a single astrocyte can transport glutamate and to quantify spatial heterogeneity in the rate of glutamate clearance kinetics within that domain. Using LSAM, we report that cortical astrocytes have an increased glutamate-responsive area following FL and that TCs have faster decay times in distal, as compared to proximal processes. Furthermore, the developmental shift from GLAST- to GLT-1-dominated clearance is disrupted following FL. These findings introduce a novel method to probe astrocyte glutamate uptake and show that neonatal cortical FL disrupts the functional maturation of cortical astrocytes.
Keywords: GLAST; GLT-1; astrocyte; freeze lesion; glutamate.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

