Synaptic plasticity and sensory-motor improvement following fibrin sealant dorsal root reimplantation and mononuclear cell therapy
- PMID: 25249946
- PMCID: PMC4158877
- DOI: 10.3389/fnana.2014.00096
Synaptic plasticity and sensory-motor improvement following fibrin sealant dorsal root reimplantation and mononuclear cell therapy
Abstract
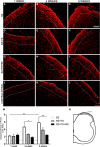
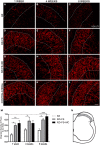
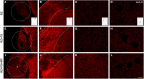
Root lesions may affect both dorsal and ventral roots. However, due to the possibility of generating further inflammation and neuropathic pain, surgical procedures do not prioritize the repair of the afferent component. The loss of such sensorial input directly disturbs the spinal circuits thus affecting the functionality of the injuried limb. The present study evaluated the motor and sensory improvement following dorsal root reimplantation with fibrin sealant (FS) plus bone marrow mononuclear cells (MC) after dorsal rhizotomy. MC were used to enhance the repair process. We also analyzed changes in the glial response and synaptic circuits within the spinal cord. Female Lewis rats (6-8 weeks old) were divided in three groups: rhizotomy (RZ group), rhizotomy repaired with FS (RZ+FS group) and rhizotomy repaired with FS and MC (RZ+FS+MC group). The behavioral tests electronic von-Frey and Walking track test were carried out. For immunohistochemistry we used markers to detect different synapse profiles as well as glial reaction. The behavioral results showed a significant decrease in sensory and motor function after lesion. The reimplantation decreased glial reaction and improved synaptic plasticity of afferent inputs. Cell therapy further enhanced the rewiring process. In addition, both reimplanted groups presented twice as much motor control compared to the non-treated group. In conclusion, the reimplantation with FS and MC is efficient and may be considered an approach to improve sensory-motor recovery following dorsal rhizotomy.
Keywords: dorsal root rhizotomy; fibrin sealant; mononuclear cells; motor control; sensory recovery.
Figures









Similar articles
-
Reflex arc recovery after spinal cord dorsal root repair with platelet rich plasma (PRP).Brain Res Bull. 2019 Oct;152:212-224. doi: 10.1016/j.brainresbull.2019.07.024. Epub 2019 Jul 24. Brain Res Bull. 2019. PMID: 31351157
-
Long-term spinal ventral root reimplantation, but not bone marrow mononuclear cell treatment, positively influences ultrastructural synapse recovery and motor axonal regrowth.Int J Mol Sci. 2014 Oct 28;15(11):19535-51. doi: 10.3390/ijms151119535. Int J Mol Sci. 2014. PMID: 25353176 Free PMC article.
-
Influence of delivery method on neuroprotection by bone marrow mononuclear cell therapy following ventral root reimplantation with fibrin sealant.PLoS One. 2014 Aug 26;9(8):e105712. doi: 10.1371/journal.pone.0105712. eCollection 2014. PLoS One. 2014. PMID: 25157845 Free PMC article.
-
Spinal Reflex Recovery after Dorsal Rhizotomy and Repair with Platelet-Rich Plasma (PRP) Gel Combined with Bioengineered Human Embryonic Stem Cells (hESCs).Stem Cells Int. 2020 Oct 29;2020:8834360. doi: 10.1155/2020/8834360. eCollection 2020. Stem Cells Int. 2020. PMID: 33178285 Free PMC article.
-
Endogenous neurotrophins and plasticity following spinal deafferentation.Exp Neurol. 2012 May;235(1):70-7. doi: 10.1016/j.expneurol.2010.12.021. Epub 2010 Dec 30. Exp Neurol. 2012. PMID: 21195072 Review.
Cited by
-
A new heterologous fibrin sealant as a scaffold to cartilage repair-Experimental study and preliminary results.Exp Biol Med (Maywood). 2016 Jul;241(13):1410-5. doi: 10.1177/1535370215597192. Epub 2015 Aug 10. Exp Biol Med (Maywood). 2016. PMID: 26264444 Free PMC article.
-
Heterologous fibrin sealant derived from snake venom: from bench to bedside - an overview.J Venom Anim Toxins Incl Trop Dis. 2017 Apr 4;23:21. doi: 10.1186/s40409-017-0109-8. eCollection 2017. J Venom Anim Toxins Incl Trop Dis. 2017. PMID: 28396682 Free PMC article. Review.
-
Long-Standing Motor and Sensory Recovery following Acute Fibrin Sealant Based Neonatal Sciatic Nerve Repair.Neural Plast. 2016;2016:9028126. doi: 10.1155/2016/9028126. Epub 2016 Jun 29. Neural Plast. 2016. PMID: 27446617 Free PMC article.
-
Embryonic stem cells overexpressing high molecular weight FGF2 isoform enhance recovery of pre-ganglionic spinal root lesion in combination with fibrin biopolymer mediated root repair.Stem Cell Res Ther. 2024 Mar 5;15(1):63. doi: 10.1186/s13287-024-03676-6. Stem Cell Res Ther. 2024. PMID: 38438875 Free PMC article.
-
The Time Course of MHC-I Expression in C57BL/6J and A/J Mice Correlates with the Degree of Retrograde Gliosis in the Spinal Cord following Sciatic Nerve Crush.Cells. 2022 Nov 22;11(23):3710. doi: 10.3390/cells11233710. Cells. 2022. PMID: 36496969 Free PMC article.
References
-
- Barros L. C., Ferreira R. S., Jr., Barraviera S. R., Stolf H. O., Thomazini-Santos I. A., Mendes-Giannini M. J., et al. (2009). A new fibrin sealant from Crotalus durissus terrificus venom: applications in medicine. J. Toxicol. Environ. Health B Crit. Rev. 12, 553–571 10.1080/10937400903442514 - DOI - PubMed
-
- Barros L. C., Soares A. M., Costa F. L., Rodrigues V. M., Fuly A. L., Giglio J. R., et al. (2011). Biochemical and biological evaluation of gyroxin isolated from Crotalus durissus terrificus venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 17, 23–33 10.1590/S1678-91992011000100004 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

