Reciprocal modulation of Aβ42 aggregation by copper and homocysteine
- PMID: 25249976
- PMCID: PMC4157544
- DOI: 10.3389/fnagi.2014.00237
Reciprocal modulation of Aβ42 aggregation by copper and homocysteine
Abstract
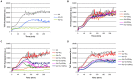
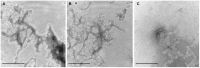
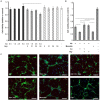
Hyperhomocysteinemia is a risk factor for Alzheimer's disease (AD). Both homocysteine (Hcy) and amyloid β (Aβ), which accumulates in the brain of AD patients, bind copper. Aim of this study was to test the hypothesis that the association of Hcy and AD results from a molecular interaction between Hcy and Aβ that is mediated by copper. We established a microtiter plate format thioflavin T aggregation assay to monitor Aβ42 fibrillization. Copper (5 μM) completely prevented Aβ42 (5 μM) fibrillization. Homocysteine in the absence of copper did not impact Aβ42 fibrillization, but physiological concentrations of Hcy (10-100 μM) attenuated the inhibitory effect of copper on Aβ42 fibril formation. These results were qualitatively confirmed by electron microscopy, which did not reveal morphological differences. To compare the toxicity of fibrillar and non-fibrillar Aβ42 exposed to copper or Hcy, rat primary cortical neurons were treated in vitro with 5 μM Aβ42 for 72 h. After incubation with 5 μM Aβ42 that had been aggregating in the absence of Hcy or copper, cell viability was reduced to 40%. Incubation with 5 μM Aβ42, in which fibril formation had been prevented or reverted by the addition of 5 μM copper, resulted in cell viability of approximately 25%. Accordingly, viability was reduced to 25% after incubation with 5 μM monomeric, i.e., non-fibrillized, Aβ42. The addition of Hcy plus copper to 5 μM Aβ42 yielded 50% viability. In conclusion, copper prevents and reverts Aβ fibril formation leading rather to formation of lower order oligomers or amorphous aggregates, and Hcy reduces these effects. Such mechanisms may explain the association of hyperhomocysteinemia and AD, leading to novel therapeutic strategies in the prevention and treatment of this disease.
Keywords: Alzheimer’s disease; Aβ; copper; cytotoxicity; homocysteine; primary neurons.
Figures


Similar articles
-
The effect of Cu(2+) and Zn(2+) on the Aβ42 peptide aggregation and cellular toxicity.Metallomics. 2013 Nov;5(11):1529-36. doi: 10.1039/c3mt00161j. Metallomics. 2013. PMID: 23995980 Free PMC article.
-
Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation.Neurotoxicology. 2009 Nov;30(6):986-95. doi: 10.1016/j.neuro.2009.08.013. Epub 2009 Sep 8. Neurotoxicology. 2009. PMID: 19744518
-
A traditional Chinese Medicine, YXQN, Reduces Amyloid-induced Cytotoxicity by Inhibiting Aβ42 Aggregation and Fibril Formation.Curr Pharm Des. 2020;26(7):780-789. doi: 10.2174/1381612826666200207120602. Curr Pharm Des. 2020. PMID: 32031066
-
Hormetic-Like Effects of L-Homocysteine on Synaptic Structure, Function, and Aβ Aggregation.Pharmaceuticals (Basel). 2020 Feb 2;13(2):24. doi: 10.3390/ph13020024. Pharmaceuticals (Basel). 2020. PMID: 32024240 Free PMC article.
-
Effects of Cu(II) on the aggregation of amyloid-β.J Biol Inorg Chem. 2019 Dec;24(8):1197-1215. doi: 10.1007/s00775-019-01727-5. Epub 2019 Oct 10. J Biol Inorg Chem. 2019. PMID: 31602542 Review.
Cited by
-
Implications of peptide assemblies in amyloid diseases.Chem Soc Rev. 2017 Oct 30;46(21):6492-6531. doi: 10.1039/c7cs00372b. Chem Soc Rev. 2017. PMID: 28702523 Free PMC article. Review.
-
Icariside II Ameliorates Cognitive Impairments Induced by Chronic Cerebral Hypoperfusion by Inhibiting the Amyloidogenic Pathway: Involvement of BDNF/TrkB/CREB Signaling and Up-Regulation of PPARα and PPARγ in Rats.Front Pharmacol. 2018 Oct 23;9:1211. doi: 10.3389/fphar.2018.01211. eCollection 2018. Front Pharmacol. 2018. PMID: 30405422 Free PMC article.
-
A Bright, Nontoxic, and Non-aggregating red Fluorescent Protein for Long-Term Labeling of Fine Structures in Neurons.Front Cell Dev Biol. 2022 Jun 29;10:893468. doi: 10.3389/fcell.2022.893468. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35846353 Free PMC article.
-
Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions.Mol Cell Biochem. 2017 Jan;425(1-2):85-93. doi: 10.1007/s11010-016-2864-1. Epub 2016 Nov 1. Mol Cell Biochem. 2017. PMID: 27804051
-
Rationally Designed Pentapeptide Analogs of Aβ19-23 Fragment as Potent Inhibitors of Aβ42 Aggregation.Molecules. 2025 May 7;30(9):2071. doi: 10.3390/molecules30092071. Molecules. 2025. PMID: 40363876 Free PMC article.
References
-
- Agnati L. F., Genedani S., Leo G., Forni A., Woods A. S., Filaferro M., et al. (2007). Abeta peptides as one of the crucial volume transmission signals in the trophic units and their interactions with homocysteine. Physiological implications and relevance for Alzheimer’s disease. J. Neural Transm. 114, 21–31 10.1007/s00702-006-0564-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

