Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage
- PMID: 25249983
- PMCID: PMC4157603
- DOI: 10.3389/fphys.2014.00340
Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage
Abstract
Introduction: Acute kidney injury (AKI) and intestinal injury negatively impact patient outcome after cardiac surgery. Enhanced nitric oxide (NO) consumption due to intraoperative intravascular hemolysis, may play an important role in this setting. This study investigated the impact of hemolysis on plasma NO consumption, AKI, and intestinal tissue damage, after cardiac surgery.
Methods: Hemolysis (by plasma extracellular (free) hemoglobin; fHb), plasma NO-consumption, plasma fHb-binding capacity by haptoglobin (Hp), renal tubular injury (using urinary N-Acetyl-β-D-glucosaminidase; NAG), intestinal mucosal injury (through plasma intestinal fatty acid binding protein; IFABP), and AKI were studied in patients undergoing off-pump cardiac surgery (OPCAB, N = 7), on-pump coronary artery bypass grafting (CABG, N = 30), or combined CABG and valve surgery (CABG+Valve, N = 30).
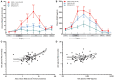
Results: FHb plasma levels and NO-consumption significantly increased, while plasma Hp concentrations significantly decreased in CABG and CABG+Valve patients (p < 0.0001) during surgery. The extent of hemolysis and NO-consumption correlated significantly (r (2) = 0.75, p < 0.0001). Also, NAG and IFABP increased in both groups (p < 0.0001, and p < 0.001, respectively), and both were significantly associated with hemolysis (R s = 0.70, p < 0.0001, and R s = 0.26, p = 0.04, respectively) and NO-consumption (Rs = 0.55, p = 0.002, and R s = 0.41, p = 0.03, respectively), also after multivariable logistic regression analysis. OPCAB patients did not show increased fHb, NO-consumption, NAG, or IFABP levels. Patients suffering from AKI (N = 9, 13.4%) displayed significantly higher fHb and NAG levels already during surgery compared to non-AKI patients.
Conclusions: Hemolysis appears to be an important contributor to postoperative kidney injury and intestinal mucosal damage, potentially by limiting NO-bioavailability. This observation offers a novel diagnostic and therapeutic target to improve patient outcome after cardiothoracic surgery.
Keywords: acute kidney injury; cardiopulmonary bypass; hemolysis; intestinal fatty acid binding protein; nitric oxide.
Figures


References
-
- Baskurt O. K., Uyuklu M., Meiselman H. J. (2004). Protection of erythrocytes from sub-hemolytic mechanical damage by nitric oxide mediated inhibition of potassium leakage. Biorheology 41, 79–89 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

