Feedbacks between protistan single-cell activity and bacterial physiological structure reinforce the predator/prey link in microbial foodwebs
- PMID: 25250018
- PMCID: PMC4155813
- DOI: 10.3389/fmicb.2014.00453
Feedbacks between protistan single-cell activity and bacterial physiological structure reinforce the predator/prey link in microbial foodwebs
Abstract
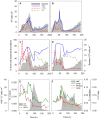
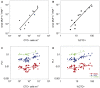
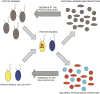
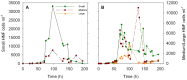
The trophic interactions between bacteria and their main predators, the heterotrophic nanoflagellates (HNFs), play a key role in the structuring and functioning of aquatic microbial food webs. Grazing regulation of bacterial communities, both of biomass and community structure, have been frequently reported. Additionally, bottom-up responses of the HNF at the population level (numerical responses) have also been extensively described. However, the functional response of HNF at the single-cell level has not been well explored. In this study, we concurrently measured the physiological structure of bacterial communities and HNF single-cell activities during re-growth cultures of natural aquatic communities. We found that changes in the abundance and proportion of the preferred, highly active bacterial prey, caused by the feeding activity of their predators (HNF), induced a negative feedback effect on the single-cell activity of these HNF. These shifts in the specific cellular activity of HNF occur at a much shorter time scale than population level shifts in flagellate abundance, and offer a complementary mechanism to explain not only the tight coupling between bacteria and HNF, but also the relative constancy of bacterial abundance in aquatic ecosystems.
Keywords: aquatic food webs; feedbacks; functional response; heterotrophic bacteria; heterotrophic nanoflagellates; predator-prey link; single-cell activity.
Figures

References
-
- Andersen P., Sorensen H. M. (1986). Population-dynamics and trophic coupling in pelagic microorganisms in eutrophic coastal waters. Mar. Ecol. Prog. Ser. 33 99–109 10.3354/meps033099 - DOI
-
- Andersson A., Larsson U., Hagstrom A. (1986). Size-selective grazing by a microflagellate on pelagic bacteria. Mar. Ecol. Prog. Ser. 33 51–57 10.3354/meps033051 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

