Enzyme-responsive nanomaterials for controlled drug delivery
- PMID: 25251024
- PMCID: PMC4425417
- DOI: 10.1039/c4nr04249b
Enzyme-responsive nanomaterials for controlled drug delivery
Abstract
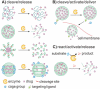
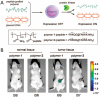
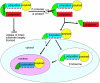
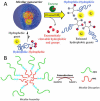
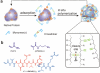
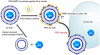
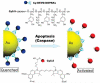
Enzymes underpin physiological function and exhibit dysregulation in many disease-associated microenvironments and aberrant cell processes. Exploiting altered enzyme activity and expression for diagnostics, drug targeting, and drug release is tremendously promising. When combined with booming research in nanobiotechnology, enzyme-responsive nanomaterials used for controlled drug release have achieved significant development and have been studied as an important class of drug delivery strategies in nanomedicine. In this review, we describe enzymes such as proteases, phospholipases and oxidoreductases that serve as delivery triggers. Subsequently, we explore recently developed enzyme-responsive nanomaterials with versatile applications for extracellular and intracellular drug delivery. We conclude by discussing future opportunities and challenges in this area.
Figures
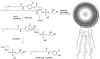





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

