Axl activates autocrine transforming growth factor-β signaling in hepatocellular carcinoma
- PMID: 25251599
- PMCID: PMC4450343
- DOI: 10.1002/hep.27492
Axl activates autocrine transforming growth factor-β signaling in hepatocellular carcinoma
Abstract
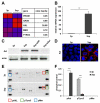
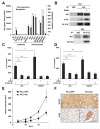
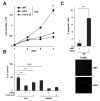
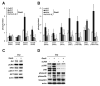
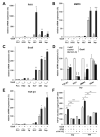
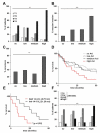
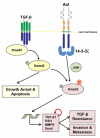
In hepatocellular carcinoma (HCC), intrahepatic metastasis frequently correlates with epithelial to mesenchymal transition (EMT) of malignant hepatocytes. Several mechanisms have been identified to be essentially involved in hepatocellular EMT, among them transforming growth factor (TGF)-β signaling. Here we show the up-regulation and activation of the receptor tyrosine kinase Axl in EMT-transformed hepatoma cells. Knockdown of Axl expression resulted in abrogation of invasive and transendothelial migratory abilities of mesenchymal HCC cells in vitro and Axl overexpression-induced metastatic colonization of epithelial hepatoma cells in vivo. Importantly, Axl knockdown severely impaired resistance to TGF-β-mediated growth inhibition. Analysis of the Axl interactome revealed binding of Axl to 14-3-3ζ, which is essentially required for Axl-mediated cell invasion, transendothelial migration, and resistance against TGF-β. Axl/14-3-3ζ signaling caused phosphorylation of Smad3 linker region (Smad3L) at Ser213, resulting in the up-regulation of tumor-progressive TGF-β target genes such as PAI1, MMP9, and Snail as well as augmented TGF-β1 secretion in mesenchymal HCC cells. Accordingly, high Axl expression in HCC patient samples correlated with elevated vessel invasion of HCC cells, higher risk of tumor recurrence after liver transplantation, strong phosphorylation of Smad3L, and lower survival. In addition, elevated expression of both Axl and 14-3-3ζ showed strongly reduced survival of HCC patients.
Conclusion: Our data suggest that Axl/14-3-3ζ signaling is central for TGF-β-mediated HCC progression and a promising target for HCC therapy.
© 2014 by the American Association for the Study of Liver Diseases.
Figures
Comment in
-
When good transforming growth factor-β turns bad in hepatocellular carcinoma: Axl takes the stage.Hepatology. 2015 Mar;61(3):759-61. doi: 10.1002/hep.27624. Epub 2015 Jan 28. Hepatology. 2015. PMID: 25429775 No abstract available.
References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. - PubMed
-
- Reichl P, Haider C, Grubinger M, Mikulits W. TGF-beta in epithelial to mesenchymal transition and metastasis of liver carcinoma. Curr Pharm Des. 2012;18:4135–4147. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Giannelli G, Villa E, Lahn M. Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74:1890–1894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
