Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination
- PMID: 25253341
- PMCID: PMC4248988
- DOI: 10.1128/JVI.02086-14
Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination
Abstract
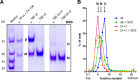
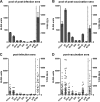
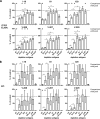
Tick-borne encephalitis (TBE) virus is an important human-pathogenic flavivirus endemic in large parts of Europe and Central and Eastern Asia. Neutralizing antibodies specific for the viral envelope protein E are believed to mediate long-lasting protection after natural infection and vaccination. To study the specificity and individual variation of human antibody responses, we developed immunoassays with recombinant antigens representing viral surface protein domains and domain combinations. These allowed us to dissect and quantify antibody populations of different fine specificities in sera of TBE patients and vaccinees. Postinfection and postvaccination sera both displayed strong individual variation of antibody titers as well as the relative proportions of antibodies to different domains of E, indicating that the immunodominance patterns observed were strongly influenced by individual-specific factors. The contributions of these antibody populations to virus neutralization were quantified by serum depletion analyses and revealed a significantly biased pattern. Antibodies to domain III, in contrast to what was found in mouse immunization studies with TBE and other flaviviruses, did not play any role in the human neutralizing antibody response, which was dominated by antibodies to domains I and II. Importantly, most of the neutralizing activity could be depleted from sera by a dimeric soluble form of the E protein, which is the building block of the icosahedral herringbone-like shell of flaviviruses, suggesting that antibodies to more complex quaternary epitopes involving residues from adjacent dimers play only a minor role in the total response to natural infection and vaccination in humans.
Importance: Tick-borne encephalitis (TBE) virus is a close relative of yellow fever, dengue, Japanese encephalitis, and West Nile viruses and distributed in large parts of Europe and Central and Eastern Asia. Antibodies to the viral envelope protein E prevent viral attachment and entry into cells and thus mediate virus neutralization and protection from disease. However, the fine specificity and individual variation of neutralizing antibody responses are currently not known. We have therefore developed new in vitro assays for dissecting the antibody populations present in blood serum and determining their contribution to virus neutralization. In our analysis of human postinfection and postvaccination sera, we found an extensive variation of the antibody populations present in sera, indicating substantial influences of individual-specific factors that control the specificity of the antibody response. Our study provides new insights into the immune response to an important human pathogen that is of relevance for the design of novel vaccines.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Simmonds P, Becher P, Collett MS, Gould EA, Heinz FX, Meyers G, Monath T, Pletnev A, Rice CM, Stiasny K, Thiel HJ, Weiner A, Bukh J. 2011. Family Flaviviridae. In King AMQ, Adams MJ, Lefkowitz EJ, Carstens EB. (ed), Virus taxonomy, vol IX Report of the International Committee on Taxonomy of Viruses Elsevier Academic Press, San Diego, CA.
-
- Monath TP, Gershman M, Staples JE, Barrett ADT. 2013. Yellow fever vaccine, p 870–968 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed. Elsevier Saunders, Philadelphia, PA.
-
- Halstead SB, Jacobson J, Dubischar-Kastner K. 2013. Japanese encephalitis vaccine, p 312–351 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed. Elsevier Saunders, Philadelphia, PA.
-
- Barrett PN, Portsmouth D, Ehrlich HJ. 2013. Tick-borne encephalitis virus vaccines, p 773–788 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed. Elsevier Saunders, Philadelphia, PA.
-
- Halstead SB, Thomas SJ. 2013. Dengue vaccines, p 1042–1051 In Plotkin SA, Orenstein WA, Offit PA. (ed), Vaccines, 6th ed. Elsevier Saunders, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

