Vaccinia virus mutations in the L4R gene encoding a virion structural protein produce abnormal mature particles lacking a nucleocapsid
- PMID: 25253347
- PMCID: PMC4249119
- DOI: 10.1128/JVI.02126-14
Vaccinia virus mutations in the L4R gene encoding a virion structural protein produce abnormal mature particles lacking a nucleocapsid
Abstract
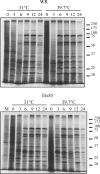
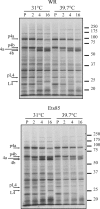
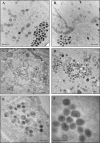
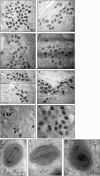
Electron micrographs from the 1960s revealed the presence of an S-shaped tubular structure in the center of the vaccinia virion core. Recently, we showed that packaging of virus transcription enzymes is necessary for the formation of the tubular structure, suggesting that the structure is equivalent to a nucleocapsid. Based on this study and on what is known about nucleocapsids of other viruses, we hypothesized that in addition to transcription enzymes, the tubular structure also contains the viral DNA and a structural protein as a scaffold. The vaccinia virion structural protein L4 stands out as the best candidate for the role of a nucleocapsid structural protein because it is abundant, it is localized in the center of the virion core, and it binds DNA. In order to gain more insight into the structure and relevance of the nucleocapsid, we analyzed thermosensitive and inducible mutants in the L4R gene. Using a cryo-fixation method for electron microscopy (high-pressure freezing followed by freeze-substitution) to preserve labile structures like the nucleocapsid, we were able to demonstrate that in the absence of functional L4, mature particles with defective internal structures are produced under nonpermissive conditions. These particles do not contain a nucleocapsid. In addition, the core wall of these virions is abnormal. This suggests that the nucleocapsid interacts with the core wall and that the nucleocapsid structure might be more complex than originally assumed.
Importance: The vaccinia virus nucleocapsid has been neglected since the 1960s due to a lack of electron microscopy techniques to preserve this labile structure. With the advent of cryo-fixation techniques, like high-pressure freezing/freeze-substitution, we are now able to consistently preserve and visualize the nucleocapsid. Because vaccinia virus early transcription is coupled to the viral core structure, detailing the structure of the nucleocapsid is indispensable for determining the mechanisms of vaccinia virus core-directed transcription. The present study represents our second attempt to understand the structure and biological significance of the nucleocapsid. We demonstrate the importance of the protein L4 for the formation of the nucleocapsid and reveal in addition that the nucleocapsid and the core wall may be associated, suggesting a higher level of complexity of the nucleocapsid than predicted. In addition, we prove the utility of high-pressure freezing in preserving the vaccinia virus nucleocapsid.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Moss B. 2007. Poxviridae: the viruses and their replication, 2906–2945 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

