Drosophila C virus systemic infection leads to intestinal obstruction
- PMID: 25253354
- PMCID: PMC4249126
- DOI: 10.1128/JVI.02320-14
Drosophila C virus systemic infection leads to intestinal obstruction
Abstract
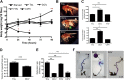
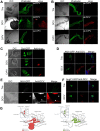
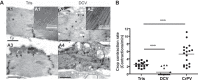
Drosophila C virus (DCV) is a positive-sense RNA virus belonging to the Dicistroviridae family. This natural pathogen of the model organism Drosophila melanogaster is commonly used to investigate antiviral host defense in flies, which involves both RNA interference and inducible responses. Although lethality is used routinely as a readout for the efficiency of the antiviral immune response in these studies, virus-induced pathologies in flies still are poorly understood. Here, we characterize the pathogenesis associated with systemic DCV infection. Comparison of the transcriptome of flies infected with DCV or two other positive-sense RNA viruses, Flock House virus and Sindbis virus, reveals that DCV infection, unlike those of the other two viruses, represses the expression of a large number of genes. Several of these genes are expressed specifically in the midgut and also are repressed by starvation. We show that systemic DCV infection triggers a nutritional stress in Drosophila which results from intestinal obstruction with the accumulation of peritrophic matrix at the entry of the midgut and the accumulation of the food ingested in the crop, a blind muscular food storage organ. The related virus cricket paralysis virus (CrPV), which efficiently grows in Drosophila, does not trigger this pathology. We show that DCV, but not CrPV, infects the smooth muscles surrounding the crop, causing extensive cytopathology and strongly reducing the rate of contractions. We conclude that the pathogenesis associated with systemic DCV infection results from the tropism of the virus for an important organ within the foregut of dipteran insects, the crop.
Importance: DCV is one of the few identified natural viral pathogens affecting the model organism Drosophila melanogaster. As such, it is an important virus for the deciphering of host-virus interactions in insects. We characterize here the pathogenesis associated with DCV infection in flies and show that it results from the tropism of the virus for an essential but poorly characterized organ in the digestive tract, the crop. Our results may have relevance for other members of the Dicistroviridae, some of which are pathogenic to beneficial or pest insect species.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

