Early evolution of limb regeneration in tetrapods: evidence from a 300-million-year-old amphibian
- PMID: 25253458
- PMCID: PMC4211449
- DOI: 10.1098/rspb.2014.1550
Early evolution of limb regeneration in tetrapods: evidence from a 300-million-year-old amphibian
Abstract
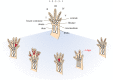
Salamanders are the only tetrapods capable of fully regenerating their limbs throughout their entire lives. Much data on the underlying molecular mechanisms of limb regeneration have been gathered in recent years allowing for new comparative studies between salamanders and other tetrapods that lack this unique regenerative potential. By contrast, the evolution of animal regeneration just recently shifted back into focus, despite being highly relevant for research designs aiming to unravel the factors allowing for limb regeneration. We show that the 300-million-year-old temnospondyl amphibian Micromelerpeton, a distant relative of modern amphibians, was already capable of regenerating its limbs. A number of exceptionally well-preserved specimens from fossil deposits show a unique pattern and combination of abnormalities in their limbs that is distinctive of irregular regenerative activity in modern salamanders and does not occur as variants of normal limb development. This demonstrates that the capacity to regenerate limbs is not a derived feature of modern salamanders, but may be an ancient feature of non-amniote tetrapods and possibly even shared by all bony fish. The finding provides a new framework for understanding the evolution of regenerative capacity of paired appendages in vertebrates in the search for conserved versus derived molecular mechanisms of limb regeneration.
Keywords: Dissorophoidea; Palaeozoic; Temnospondyli; amphibian; fossil; limb regeneration.
Figures


References
-
- Gardiner DM, Bryant SV. 2007. Tetrapod limb regeneration. In Fins into limbs (ed. Hall BK.), pp. 163–182. Chicago, IL: University of Chicago Press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
