Treatment of heterotopic ossification through remote ATP hydrolysis
- PMID: 25253675
- PMCID: PMC4353731
- DOI: 10.1126/scitranslmed.3008810
Treatment of heterotopic ossification through remote ATP hydrolysis
Abstract
Heterotopic ossification (HO) is the pathologic development of ectopic bone in soft tissues because of a local or systemic inflammatory insult, such as burn injury or trauma. In HO, mesenchymal stem cells (MSCs) are inappropriately activated to undergo osteogenic differentiation. Through the correlation of in vitro assays and in vivo studies (dorsal scald burn with Achilles tenotomy), we have shown that burn injury enhances the osteogenic potential of MSCs and causes ectopic endochondral heterotopic bone formation and functional contractures through bone morphogenetic protein-mediated canonical SMAD signaling. We further demonstrated a prevention strategy for HO through adenosine triphosphate (ATP) hydrolysis at the burn site using apyrase. Burn site apyrase treatment decreased ATP, increased adenosine 3',5'-monophosphate, and decreased phosphorylation of SMAD1/5/8 in MSCs in vitro. This ATP hydrolysis also decreased HO formation and mitigated functional impairment in vivo. Similarly, selective inhibition of SMAD1/5/8 phosphorylation with LDN-193189 decreased HO formation and increased range of motion at the injury site in our burn model in vivo. Our results suggest that burn injury-exacerbated HO formation can be treated through therapeutics that target burn site ATP hydrolysis and modulation of SMAD1/5/8 phosphorylation.
Copyright © 2014, American Association for the Advancement of Science.
Figures

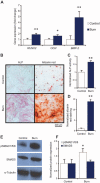
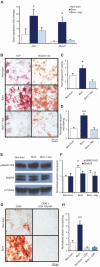


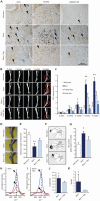
Comment in
-
Burned to the bone.Sci Transl Med. 2014 Sep 24;6(255):255fs37. doi: 10.1126/scitranslmed.3010168. Sci Transl Med. 2014. PMID: 25253672
References
-
- Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J. Surg. Orthop. Adv. 2010;19:54–61. - PubMed
-
- Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J. Bone Joint Surg. Am. 2010;92(Suppl. 2):74–89. - PubMed
-
- Hunt JL, Arnoldo BD, Kowalske K, Helm P, Purdue GF. Heterotopic ossification revisited: A 21-year surgical experience. J. Burn Care Res. 2006;27:535–540. - PubMed
-
- Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J. Bone Joint Surg. Br. 2004;86:396–403. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- U54 GM062119/GM/NIGMS NIH HHS/United States
- T32-GM8256/GM/NIGMS NIH HHS/United States
- P50-GM60338/GM/NIGMS NIH HHS/United States
- R01 GM056687/GM/NIGMS NIH HHS/United States
- R01GM098350-02/GM/NIGMS NIH HHS/United States
- 1K08GM109105-01/GM/NIGMS NIH HHS/United States
- U54-GM062119/GM/NIGMS NIH HHS/United States
- R24 GM102656/GM/NIGMS NIH HHS/United States
- R01 AR055222/AR/NIAMS NIH HHS/United States
- K08 GM109105/GM/NIGMS NIH HHS/United States
- P50 GM021700/GM/NIGMS NIH HHS/United States
- R24-GM102656/GM/NIGMS NIH HHS/United States
- R01 GM098350/GM/NIGMS NIH HHS/United States
- R01 GM101401/GM/NIGMS NIH HHS/United States
- P50 GM060338/GM/NIGMS NIH HHS/United States
- T32 GM008256/GM/NIGMS NIH HHS/United States
- R01-GM56687/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

