Origins of fluorescence in evolved bacteriophytochromes
- PMID: 25253687
- PMCID: PMC4231690
- DOI: 10.1074/jbc.M114.589739
Origins of fluorescence in evolved bacteriophytochromes
Abstract
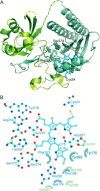
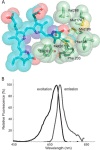
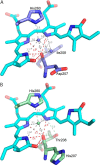
Use of fluorescent proteins to study in vivo processes in mammals requires near-infrared (NIR) biomarkers that exploit the ability of light in this range to penetrate tissue. Bacteriophytochromes (BphPs) are photoreceptors that couple absorbance of NIR light to photoisomerization, protein conformational changes, and signal transduction. BphPs have been engineered to form NIR fluorophores, including IFP1.4, Wi-Phy, and the iRFP series, initially by replacement of Asp-207 by His. This position was suggestive because its main chain carbonyl is within hydrogen-bonding distance to pyrrole ring nitrogens of the biliverdin chromophore, thus potentially functioning as a crucial transient proton sink during photoconversion. To explain the origin of fluorescence in these phytofluors, we solved the crystal structures of IFP1.4 and a comparison non-fluorescent monomeric phytochrome DrCBDmon. Met-186 and Val-288 in IFP1.4 are responsible for the formation of a tightly packed hydrophobic hub around the biliverdin D ring. Met-186 is also largely responsible for the blue-shifted IFP1.4 excitation maximum relative to the parent BphP. The structure of IFP1.4 revealed decreased structural heterogeneity and a contraction of two surface regions as direct consequences of side chain substitutions. Unexpectedly, IFP1.4 with Asp-207 reinstalled (IFPrev) has a higher fluorescence quantum yield (∼9%) than most NIR phytofluors published to date. In agreement, fluorescence lifetime measurements confirm the exceptionally long excited state lifetimes, up to 815 ps, in IFP1.4 and IFPrev. Our research helps delineate the origin of fluorescence in engineered BphPs and will facilitate the wide-spread adoption of phytofluors as biomarkers.
Keywords: Fluorescence; Photoreceptor; Phytochrome; Protein Engineering; Spectroscopy; Structural Biology.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
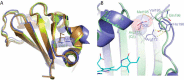
References
-
- Shimomura O., Johnson F. H., Saiga Y. (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 59, 223–239 - PubMed
-
- Jöbsis F. F. (1974) Intracellular metabolism of oxygen. Am. Rev. Respir. Dis. 110, 58–63 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

