Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein
- PMID: 25253696
- PMCID: PMC4223305
- DOI: 10.1074/jbc.M114.589069
Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein
Abstract
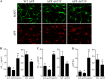
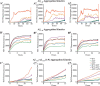
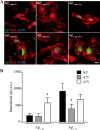
Pathogenic mutations in the amyloid precursor protein (APP) gene have been described as causing early onset familial Alzheimer disease (AD). We recently identified a rare APP variant encoding an alanine-to-threonine substitution at residue 673 (A673T) that confers protection against development of AD (Jonsson, T., Atwal, J. K., Steinberg, S., Snaedal, J., Jonsson, P. V., Bjornsson, S., Stefansson, H., Sulem, P., Gudbjartsson, D., Maloney, J., Hoyte, K., Gustafson, A., Liu, Y., Lu, Y., Bhangale, T., Graham, R. R., Huttenlocher, J., Bjornsdottir, G., Andreassen, O. A., Jönsson, E. G., Palotie, A., Behrens, T. W., Magnusson, O. T., Kong, A., Thorsteinsdottir, U., Watts, R. J., and Stefansson, K. (2012) Nature 488, 96-99). The Ala-673 residue lies within the β-secretase recognition sequence and is part of the amyloid-β (Aβ) peptide cleavage product (position 2 of Aβ). We previously demonstrated that the A673T substitution makes APP a less favorable substrate for cleavage by BACE1. In follow-up studies, we confirm that A673T APP shows reduced cleavage by BACE1 in transfected mouse primary neurons and in isogenic human induced pluripotent stem cell-derived neurons. Using a biochemical approach, we show that the A673T substitution modulates the catalytic turnover rate (V(max)) of APP by the BACE1 enzyme, without affecting the affinity (K(m)) of the APP substrate for BACE1. We also show a reduced level of Aβ(1-42) aggregation with A2T Aβ peptides, an observation not conserved in Aβ(1-40) peptides. When combined in a ratio of 1:9 Aβ(1-42)/Aβ(1-40) to mimic physiologically relevant mixtures, A2T retains a trend toward slowed aggregation kinetics. Microglial uptake of the mutant Aβ(1-42) peptides correlated with their aggregation level. Cytotoxicity of the mutant Aβ peptides was not dramatically altered. Taken together, our findings demonstrate that A673T, a protective allele of APP, reproducibly reduces amyloidogenic processing of APP and also mildly decreases Aβ aggregation. These effects could together have an additive or even synergistic impact on the risk of developing AD.
Keywords: Alzheimer Disease; Amyloid Precursor Protein (APP); Amyloid-β (AB); Neurobiology; Proteolytic Enzyme; β-Secretase 1 (BACE1).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Jonsson T., Atwal J. K., Steinberg S., Snaedal J., Jonsson P. V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., Hoyte K., Gustafson A., Liu Y., Lu Y., Bhangale T., Graham R. R., Huttenlocher J., Bjornsdottir G., Andreassen O. A., Jönsson E. G., Palotie A., Behrens T. W., Magnusson O. T., Kong A., Thorsteinsdottir U., Watts R. J., Stefansson K. (2012) A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature 488, 96–99 - PubMed
-
- Cruts M., van Duijn C. M., Backhovens H., Van den Broeck M., Wehnert A., Serneels S., Sherrington R., Hutton M., Hardy J., St George-Hyslop P. H., Hofman A., Van Broeckhoven C. (1998) Estimation of the genetic contribution of presenilin-1 and −2 mutations in a population-based study of presenile Alzheimer disease. Hum. Mol. Genet. 7, 43–51 - PubMed
-
- Hardy J., Crook R. (2001) Presenilin mutations line up along transmembrane α-helices. Neurosci. Lett. 306, 203–205 - PubMed
-
- Kauwe J. S., Jacquart S., Chakraverty S., Wang J., Mayo K., Fagan A. M., Holtzman D. M., Morris J. C., Goate A. M. (2007) Extreme cerebrospinal fluid amyloid β levels identify family with late-onset Alzheimer's disease presenilin 1 mutation. Ann. Neurol. 61, 446–453 - PubMed
-
- Cruchaga C., Haller G., Chakraverty S., Mayo K., Vallania F. L., Mitra R. D., Faber K., Williamson J., Bird T., Diaz-Arrastia R., Foroud T. M., Boeve B. F., Graff-Radford N. R., St Jean P., Lawson M., Ehm M. G., Mayeux R., Goate A. M. (2012) Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer's disease families. PLoS One 7, e31039. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

