A Tradeoff Between Accuracy and Flexibility in a Working Memory Circuit Endowed with Slow Feedback Mechanisms
- PMID: 25253801
- PMCID: PMC4585505
- DOI: 10.1093/cercor/bhu202
A Tradeoff Between Accuracy and Flexibility in a Working Memory Circuit Endowed with Slow Feedback Mechanisms
Abstract
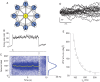
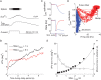
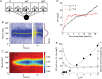
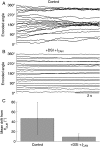
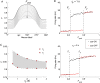
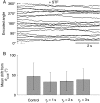
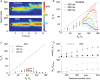
Recent studies have shown that reverberation underlying mnemonic persistent activity must be slow, to ensure the stability of a working memory system and to give rise to long neural transients capable of accumulation of information over time. Is the slower the underlying process, the better? To address this question, we investigated 3 slow biophysical mechanisms that are activity-dependent and prominently present in the prefrontal cortex: Depolarization-induced suppression of inhibition (DSI), calcium-dependent nonspecific cationic current (ICAN), and short-term facilitation. Using a spiking network model for spatial working memory, we found that these processes enhance the memory accuracy by counteracting noise-induced drifts, heterogeneity-induced biases, and distractors. Furthermore, the incorporation of DSI and ICAN enlarges the range of network's parameter values required for working memory function. However, when a progressively slower process dominates the network, it becomes increasingly more difficult to erase a memory trace. We demonstrate this accuracy-flexibility tradeoff quantitatively and interpret it using a state-space analysis. Our results supports the scenario where N-methyl-d-aspartate receptor-dependent recurrent excitation is the workhorse for the maintenance of persistent activity, whereas slow synaptic or cellular processes contribute to the robustness of mnemonic function in a tradeoff that potentially can be adjusted according to behavioral demands.
Keywords: DSI; ICAN; persistent activity; prefrontal cortex; synaptic plasticity.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Abbott LF, Regehr WG. 2004. Synaptic computation. Nature. 431:796–803. - PubMed
-
- Amit DJ. 1995. The Hebbian paradigm reintegrated: Local reverberations as internal representations. Behav Brain Sci. 18:617–626.
-
- Amit DJ, Brunel N. 1997. Model of global spontaneous activity and local structured activity during delay periods in the cerebral cortex. Cereb Cortex. 7:237–252. - PubMed
-
- Baeg E, Kim Y, Huh K, Mook-Jung I, Kim H, Jung M. 2003. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 40:177–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

