Dendritic cells from aged subjects display enhanced inflammatory responses to Chlamydophila pneumoniae
- PMID: 25253920
- PMCID: PMC4165882
- DOI: 10.1155/2014/436438
Dendritic cells from aged subjects display enhanced inflammatory responses to Chlamydophila pneumoniae
Abstract
Chlamydophila pneumoniae (CPn) is a common respiratory pathogen that causes a chronic and persistent airway infection. The elderly display an increased susceptibility and severity to this infection. However, the underlying mechanisms are not well understood. Dendritic cells (DCs) are the initiators and regulators of immune responses. Therefore, we investigated the role of DCs in the age-associated increased CPn infection in vitro in humans. Though the expression of activation markers was comparable between the two age groups, DCs from aged subjects secreted enhanced levels of proinflammatory mediators such as TNF-α and CXCL-10 in response to CPn. In contrast, the secretion of IL-10 and innate interferons, IFN-α and IFN-λ, was severely impaired in DCs from aged donors. The increased activation of DCs from aged subjects to CPn also resulted in enhanced proliferation of CD4 and CD8 T cells in a DC-T coculture. Furthermore, T cells primed with CPn-stimulated DCs from aged subjects secreted increased levels of IFN-γ and reduced levels of IL-10 compared to DCs obtained from young subjects. In summary, DCs from the elderly displayed enhanced inflammatory response to CPn which may result in airway remodeling and increase the susceptibility of the elderly to respiratory diseases such as asthma.
Figures



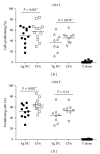
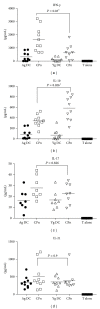
References
-
- Busse PJ, Lurslurchachai L, Sampson HA, Halm EA, Wisnivesky J. Perennial allergen-specific immunoglobulin E levels among inner-city elderly asthmatics. Journal of Asthma. 2010;47(7):781–785. - PubMed
-
- Diaz-Guzman E, Mannino DM. Airway obstructive diseases in older adults: from detection to treatment. Journal of Allergy and Clinical Immunology. 2010;126(4):702–709. - PubMed
-
- Reed CE. Asthma in the elderly: diagnosis and management. Journal of Allergy and Clinical Immunology. 2010;126(4):681–687. - PubMed
-
- Matsuse H, Tsuchida T, Fukahori S, et al. Differential airway inflammatory responses in asthma exacerbations induced by respiratory syncytial virus and influenza virus A. International Archives of Allergy and Immunology. 2013;161(4):378–382. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

