Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure
- PMID: 25254500
- PMCID: PMC4177865
- DOI: 10.1371/journal.pone.0107903
Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure
Abstract
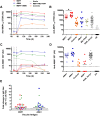
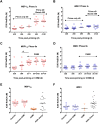
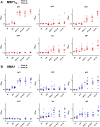
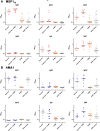
The development of protective vaccines against many difficult infectious pathogens will necessitate the induction of effective antibody responses. Here we assess humoral immune responses against two antigens from the blood-stage merozoite of the Plasmodium falciparum human malaria parasite--MSP1 and AMA1. These antigens were delivered to healthy malaria-naïve adult volunteers in Phase Ia clinical trials using recombinant replication-deficient viral vectors--ChAd63 to prime the immune response and MVA to boost. In subsequent Phase IIa clinical trials, immunized volunteers underwent controlled human malaria infection (CHMI) with P. falciparum to assess vaccine efficacy, whereby all but one volunteer developed low-density blood-stage parasitemia. Here we assess serum antibody responses against both the MSP1 and AMA1 antigens following i) ChAd63-MVA immunization, ii) immunization and CHMI, and iii) primary malaria exposure in the context of CHMI in unimmunized control volunteers. Responses were also assessed in a cohort of naturally-immune Kenyan adults to provide comparison with those induced by a lifetime of natural malaria exposure. Serum antibody responses against MSP1 and AMA1 were characterized in terms of i) total IgG responses before and after CHMI, ii) responses to allelic variants of MSP1 and AMA1, iii) functional growth inhibitory activity (GIA), iv) IgG avidity, and v) isotype responses (IgG1-4, IgA and IgM). These data provide the first in-depth assessment of the quality of adenovirus-MVA vaccine-induced antibody responses in humans, along with assessment of how these responses are modulated by subsequent low-density parasite exposure. Notable differences were observed in qualitative aspects of the human antibody responses against these malaria antigens depending on the means of their induction and/or exposure of the host to the malaria parasite. Given the continued clinical development of viral vectored vaccines for malaria and a range of other diseases targets, these data should help to guide further immuno-monitoring studies of vaccine-induced human antibody responses.
Conflict of interest statement
Figures


References
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431. - PubMed
-
- Alonso PL, Tanner M (2013) Public health challenges and prospects for malaria control and elimination. Nat Med 19: 150–155. - PubMed
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K (2008) Immunity to malaria: more questions than answers. Nat Immunol 9: 725–732. - PubMed
-
- Goodman AL, Draper SJ (2010) Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol 104: 189–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

