Hematopoietic but not endothelial cell MyD88 contributes to host defense during gram-negative pneumonia derived sepsis
- PMID: 25254554
- PMCID: PMC4177915
- DOI: 10.1371/journal.ppat.1004368
Hematopoietic but not endothelial cell MyD88 contributes to host defense during gram-negative pneumonia derived sepsis
Abstract
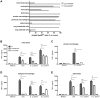
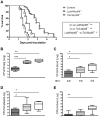
Klebsiella pneumoniae is an important cause of sepsis. The common Toll-like receptor adapter myeloid differentiation primary response gene (MyD)88 is crucial for host defense against Klebsiella. Here we investigated the role of MyD88 in myeloid and endothelial cells during Klebsiella pneumosepsis. Mice deficient for MyD88 in myeloid (LysM-Myd88(-/-)) and myeloid plus endothelial (Tie2-Myd88(-/-)) cells showed enhanced lethality and bacterial growth. Tie2-Myd88(-/-) mice reconstituted with control bone marrow, representing mice with a selective MyD88 deficiency in endothelial cells, showed an unremarkable antibacterial defense. Myeloid or endothelial cell MyD88 deficiency did not impact on lung pathology or distant organ injury during late stage sepsis, while LysM-Myd88(-/-) mice demonstrated a strongly attenuated inflammatory response in the airways early after infection. These data suggest that myeloid but not endothelial MyD88 is important for host defense during gram-negative pneumonia derived sepsis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organisation (2012) WHO causes of death 2008, Global health Observatory, World Health Organisation.
-
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, et al. (2005) Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128: 3854–3862. - PubMed
-
- Coque TM, Baquero F, Canton R (2008) Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 13 19044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

