Oligomerisation of C. elegans olfactory receptors, ODR-10 and STR-112, in yeast
- PMID: 25254556
- PMCID: PMC4177895
- DOI: 10.1371/journal.pone.0108680
Oligomerisation of C. elegans olfactory receptors, ODR-10 and STR-112, in yeast
Abstract
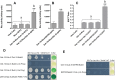
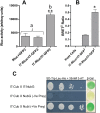
It is widely accepted that vertebrate G-Protein Coupled Receptors (GPCRs) associate with each other as homo- or hetero-dimers or higher-order oligomers. The C. elegans genome encodes hundreds of olfactory GPCRs, which may be expressed in fewer than a dozen chemosensory neurons, suggesting an opportunity for oligomerisation. Here we show, using three independent lines of evidence: co-immunoprecipitation, bioluminescence resonance energy transfer and a yeast two-hybrid assay that nematode olfactory receptors (ORs) oligomerise when heterologously expressed in yeast. Specifically, the nematode receptor ODR-10 is able to homo-oligomerise and can also form heteromers with the related nematode receptor STR-112. ODR-10 also oligomerised with the rat I7 OR but did not oligomerise with the human somatostatin receptor 5, a neuropeptide receptor. In this study, the question of functional relevance was not addressed and remains to be investigated.
Conflict of interest statement
Figures




References
-
- Marinissen MJ, Gutkind JS (2001) G-protein coupled receptors and signaling networks: emerging paradigms. Trends in Pharmacological Sciences 22: 368–376. - PubMed
-
- George SR, O’Dowd BF, Lee SP (2002) G-protein coupled receptor oligomerisation and its potential for drug discovery. Nat Rev Drug Discov 1: 808–820. - PubMed
-
- Kroeger KM, Pfleger KD, Eidne KA (2003) G-protein coupled receptor oligomerisation in neuroendocrine pathways. Front Neuroendocrinol 24: 254–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

