Reorganization of the endosomal system in Salmonella-infected cells: the ultrastructure of Salmonella-induced tubular compartments
- PMID: 25254663
- PMCID: PMC4177991
- DOI: 10.1371/journal.ppat.1004374
Reorganization of the endosomal system in Salmonella-infected cells: the ultrastructure of Salmonella-induced tubular compartments
Abstract
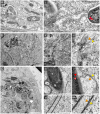
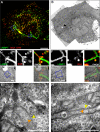
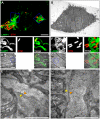
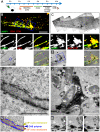
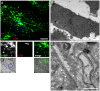
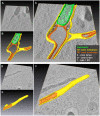
During the intracellular life of Salmonella enterica, a unique membrane-bound compartment termed Salmonella-containing vacuole, or SCV, is formed. By means of translocated effector proteins, intracellular Salmonella also induce the formation of extensive, highly dynamic membrane tubules termed Salmonella-induced filaments or SIF. Here we report the first detailed ultrastructural analyses of the SCV and SIF by electron microscopy (EM), EM tomography and live cell correlative light and electron microscopy (CLEM). We found that a subset of SIF is composed of double membranes that enclose portions of host cell cytosol and cytoskeletal filaments within its inner lumen. Despite some morphological similarities, we found that the formation of SIF double membranes is independent from autophagy and requires the function of the effector proteins SseF and SseG. The lumen of SIF network is accessible to various types of endocytosed material and our CLEM analysis of double membrane SIF demonstrated that fluid phase markers accumulate only between the inner and outer membrane of these structures, a space continual with endosomal lumen. Our work reveals how manipulation of the endosomal membrane system by an intracellular pathogen results in a unique tubular membrane compartmentalization of the host cell, generating a shielded niche permissive for intracellular proliferation of Salmonella.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Schaible UE, Haas A, editors (2009) Intracellular Niches for Microbes: A Pathogens Guide Through the Host Cell. Weinheim: Wiley-VCH.
-
- Figueira R, Holden DW (2012) Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158: 1147–1161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

