Cathepsin B in antigen-presenting cells controls mediators of the Th1 immune response during Leishmania major infection
- PMID: 25255101
- PMCID: PMC4177854
- DOI: 10.1371/journal.pntd.0003194
Cathepsin B in antigen-presenting cells controls mediators of the Th1 immune response during Leishmania major infection
Abstract
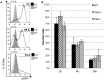
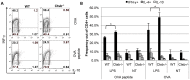
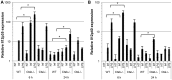
Resistance and susceptibility to Leishmania major infection in the murine model is determined by the capacity of the host to mount either a protective Th1 response or a Th2 response associated with disease progression. Previous reports involving the use of cysteine cathepsin inhibitors indicated that cathepsins B (Ctsb) and L (Ctsl) play important roles in Th1/Th2 polarization during L. major infection in both susceptible and resistant mouse strains. Although it was hypothesized that these effects are a consequence of differential patterns of antigen processing, the mechanisms underlying these differences were not further investigated. Given the pivotal roles that dendritic cells and macrophages play during Leishmania infection, we generated bone-marrow derived dendritic cells (BMDC) and macrophages (BMM) from Ctsb-/- and Ctsl-/- mice, and studied the effects of Ctsb and Ctsl deficiency on the survival of L. major in infected cells. Furthermore, the signals used by dendritic cells to instruct Th cell polarization were addressed: the expression of MHC class II and co-stimulatory molecules, and cytokine production. We found that Ctsb-/- BMDC express higher levels of MHC class II molecules than wild-type (WT) and Ctsl-/- BMDC, while there were no significant differences in the expression of co-stimulatory molecules between cathepsin-deficient and WT cells. Moreover, both BMDC and BMM from Ctsb-/- mice significantly up-regulated the levels of interleukin 12 (IL-12) expression, a key Th1-inducing cytokine. These findings indicate that Ctsb-/- BMDC display more pro-Th1 properties than their WT and Ctsl-/- counterparts, and therefore suggest that Ctsb down-regulates the Th1 response to L. major. Moreover, they propose a novel role for Ctsb as a regulator of cytokine expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- World Health Organization (2010) Control of the leishmaniasis: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases. Geneva: World Health Organization 949: : 949.
-
- World Health Organization (2012) Leishmaniasis: worldwide epidemiological and drug access update. WHO. Available: http://www.who.int/leishmaniasis/resources/Leishmaniasis_worldwide_epide....
-
- Mitchell GF, Anders RF, Brown GV, Handman E, Roberts-Thomson IC, et al. (1982) Analysis of infection characteristics and antiparasite immune responses in resistant compared with susceptible hosts. Immunol Rev 61: 137–188. - PubMed
-
- Stebut von E (2007) Immunology of cutaneous leishmaniasis: the role of mast cells, phagocytes and dendritic cells for protective immunity. Eur J Dermatol 17: 115–122. - PubMed
-
- Moll H, Fuchs H, Blank C, Röllinghoff M (1993) Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol 23: 1595–1601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

