Design, synthesis and the biological evaluation of new 1,3-thiazolidine-4-ones based on the 4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one scaffold
- PMID: 25255761
- PMCID: PMC6270961
- DOI: 10.3390/molecules190913824
Design, synthesis and the biological evaluation of new 1,3-thiazolidine-4-ones based on the 4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one scaffold
Abstract
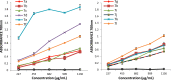
New thiazolidine-4-one derivatives based on the 4-aminophenazone (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one) scaffold have been synthesized as potential anti-inflammatory drugs. The pyrazoline derivatives are known especially for their antipyretic, analgesic and anti-inflammatory effects, but recently there were synthesized new compounds with important antioxidant, antiproliferative, anticancer and antidiabetic activities. The beneficial effects of these compounds are explained by nonselective inhibition of cyclooxygenase izoenzymes, but also by their potential scavenging ability for reactive oxygen and nitrogen species. The structure of the new compounds was proved using spectroscopic methods (FR-IR, 1H-NMR, 13C-NMR, MS). The in vitro antioxidant potential of the synthesized compounds was evaluated according to the ferric reducing antioxidant power, phosphomolydenum reducing antioxidant power, DPPH and ABTS radical scavenging assays. The chemical modulation of 4-aminophenazone (6) through linkage to thiazolidine-propanoic acid derivatives 5a-l led to improved antioxidant potential, all derivatives 7a-l being more active than phenazone. The most active compounds are the derivatives 7e, and 7k, which showed the higher antioxidant effect depending on the antioxidant assay considered.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jamwal A., Javed A., Bhardwaj V. A review on pyrazole derivatives of pharmacological potential. J. Pharm. BioSci. 2013;3:114–123.
-
- El-Hawash S., Badawey E.-S., El-Ashmawey I. Nonsteroidal antiinflammatory agents-part 2. Antiinflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin-5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur. J. Med. Chem. 2006;41:155–165. doi: 10.1016/j.ejmech.2005.09.006. - DOI - PubMed
-
- El-Sayed M.A., Abdel-Aziz N.I., Abdel-Aziz A.A., El-Azab A.S., ElTahir K.E. Synthesis, biological evaluation and molecular modeling study of pyrazole and pyrazoline derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. 2012;20:3306–3316. doi: 10.1016/j.bmc.2012.03.044. - DOI - PubMed
-
- Fushimi N., Fujikura H., Shiohara H., Teranishi H., Shimizu K., Yonekubo S., Ohno K., Miyagi T., Itoh F., Shibazaki T., et al. Structure-activity relationship studies of 4-benzyl-1H-pyrazol-3-yl β-d-glucopyranoside derivativesas potent and selective sodium glucose co-transporter 1 (SGLT1) inhibitors with therapeutic activity on postprandial hyperglycemia. Bioorg. Med. Chem. 2012;20:6598–6612. doi: 10.1016/j.bmc.2012.09.037. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

