Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats
- PMID: 25255824
- PMCID: PMC4301696
- DOI: 10.1111/bph.12951
Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats
Abstract
Background and purpose: Synthetic cathinones, commonly referred to as 'bath salts', are a group of amphetamine-like drugs gaining popularity worldwide. 4-Methylmethcathinone (mephedrone, MEPH) is the most commonly abused synthetic cathinone in the UK, and exerts its effects by acting as a substrate-type releaser at monoamine transporters. Similar to other cathinone-related compounds, MEPH has a chiral centre and exists stably as two enantiomers: R-mephedrone (R-MEPH) and S-mephedrone (S-MEPH).
Experimental approach: Here, we provide the first investigation into the neurochemical and behavioural effects of R-MEPH and S-MEPH. We analysed both enantiomers in rat brain synaptosome neurotransmitter release assays and also investigated their effects on locomotor activity (e.g. ambulatory activity and repetitive movements), behavioural sensitization and reward.
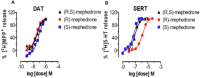
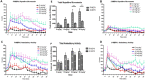
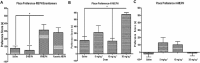
Key results: Both enantiomers displayed similar potency as substrates (i.e. releasers) at dopamine transporters, but R-MEPH was much less potent than S-MEPH as a substrate at 5-HT transporters. Locomotor activity was evaluated in acute and repeated administration paradigms, with R-MEPH producing greater repetitive movements than S-MEPH across multiple doses. After repeated drug exposure, only R-MEPH produced sensitization of repetitive movements. R-MEPH produced a conditioned place preference whereas S-MEPH did not. Lastly, R-MEPH and S-MEPH produced biphasic profiles in an assay of intracranial self-stimulation (ICSS), but R-MEPH produced greater ICSS facilitation than S-MEPH.
Conclusions and implications: Our data are the first to demonstrate stereospecific effects of MEPH enantiomers and suggest that the predominant dopaminergic actions of R-MEPH (i.e. the lack of serotonergic actions) render this stereoisomer more stimulant-like when compared with S-MEPH. This hypothesis warrants further study.
© 2014 The British Pharmacological Society.
Figures


References
-
- Arnold EB, Molinoff PB, Rutledge CO. The release of endogenous norepinephrine and dopamine from cerebral cortex by amphetamine. J Pharmacol Exp Ther. 1977;202:544–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

