Stimulated reporting: the impact of US food and drug administration-issued alerts on the adverse event reporting system (FAERS)
- PMID: 25255848
- PMCID: PMC4206770
- DOI: 10.1007/s40264-014-0225-0
Stimulated reporting: the impact of US food and drug administration-issued alerts on the adverse event reporting system (FAERS)
Abstract
Background: The US Food and Drug Administration (FDA) uses the Adverse Event Reporting System (FAERS) to support post-marketing safety surveillance programs. Currently, almost one million case reports are submitted to FAERS each year, making it a vast repository of drug safety information. Sometimes cited as a limitation of FAERS, however, is the assumption that "stimulated reporting" of adverse events (AEs) occurs in response to warnings, alerts, and label changes that are issued by the FDA.
Objective: To determine the extent of "stimulated reporting" in the modern-day FAERS database.
Methods: One hundred drugs approved by the FDA between 2001 and 2010 were included in this analysis. FDA alerts were obtained by a comprehensive search of the FDA's MedWatch and main websites. Publicly available FAERS data were used to assess the "primary suspect" AE reporting pattern for up to four quarters before, and after, the issuance of an FDA alert.
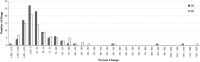
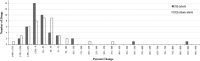
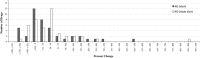
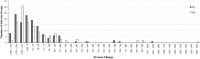
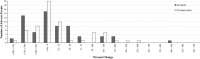
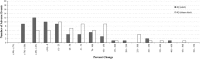
Results: A few drugs did demonstrate "stimulated reporting" trends. A majority of the drugs, however, showed little evidence for significant reporting changes associated with the issuance of alerts. When we compared the percentage changes in reporting after an FDA alert with those after a sham "control alert", the overall reporting trends appeared to be quite similar. Of 100 drugs analyzed for short-term reporting trends, 21 real alerts and 25 sham alerts demonstrated an increase (greater than or equal to 1 %) in reporting. The long-term analysis of 91 drugs showed that 24 real alerts and 28 sham alerts demonstrated a greater than or equal to 1 % increase.
Conclusions: Our results suggest that most of modern day FAERS reporting is not significantly affected by the issuance of FDA alerts.
Figures
References
-
- FDA. Safety information: Vioxx (rofecoxib). 2002. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuma... Accessed August 2014.
-
- FDA. Follow-up to the November 2009 early communication about an ongoing safety review of sibutramine, Marketed as Meridia. 2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa... Accessed August 2014.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

