Interactions between calcium and alpha-synuclein in neurodegeneration
- PMID: 25256602
- PMCID: PMC4192672
- DOI: 10.3390/biom4030795
Interactions between calcium and alpha-synuclein in neurodegeneration
Abstract
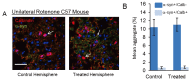
In Parkinson's disease and some atypical Parkinson's syndromes, aggregation of the α-synuclein protein (α-syn) has been linked to neurodegeneration. Many triggers for pathological α-syn aggregation have been identified, including port-translational modifications, oxidative stress and raised metal ions, such as Ca2+. Recently, it has been found using cell culture models that transient increases of intracellular Ca2+ induce cytoplasmic α-syn aggregates. Ca2+-dependent α-syn aggregation could be blocked by the Ca2+ buffering agent, BAPTA-AM, or by the Ca2+ channel blocker, Trimethadione. Furthermore, a greater proportion of cells positive for aggregates occurred when both raised Ca2+ and oxidative stress were combined, indicating that Ca2+ and oxidative stress cooperatively promote α-syn aggregation. Current on-going work using a unilateral mouse lesion model of Parkinson's disease shows a greater proportion of calbindin-positive neurons survive the lesion, with intracellular α-syn aggregates almost exclusively occurring in calbindin-negative neurons. These and other recent findings are reviewed in the context of neurodegenerative pathologies and suggest an association between raised Ca2+, α-syn aggregation and neurotoxicity.
Figures


References
-
- Radford R., Wong M.B., Pountney D.L. Neurodegenerative aspects of multiple system atrophy. In: Kostrzewa R.M., editor. Handbook of Neurotoxicity. Springer; New York, NY, USA: 2014. pp. 2157–2180.
-
- Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J.T., Schöls L., Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. - PubMed
-
- Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

