Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation
- PMID: 25256700
- PMCID: PMC4189683
- DOI: 10.1186/s12974-014-0167-6
Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation
Abstract
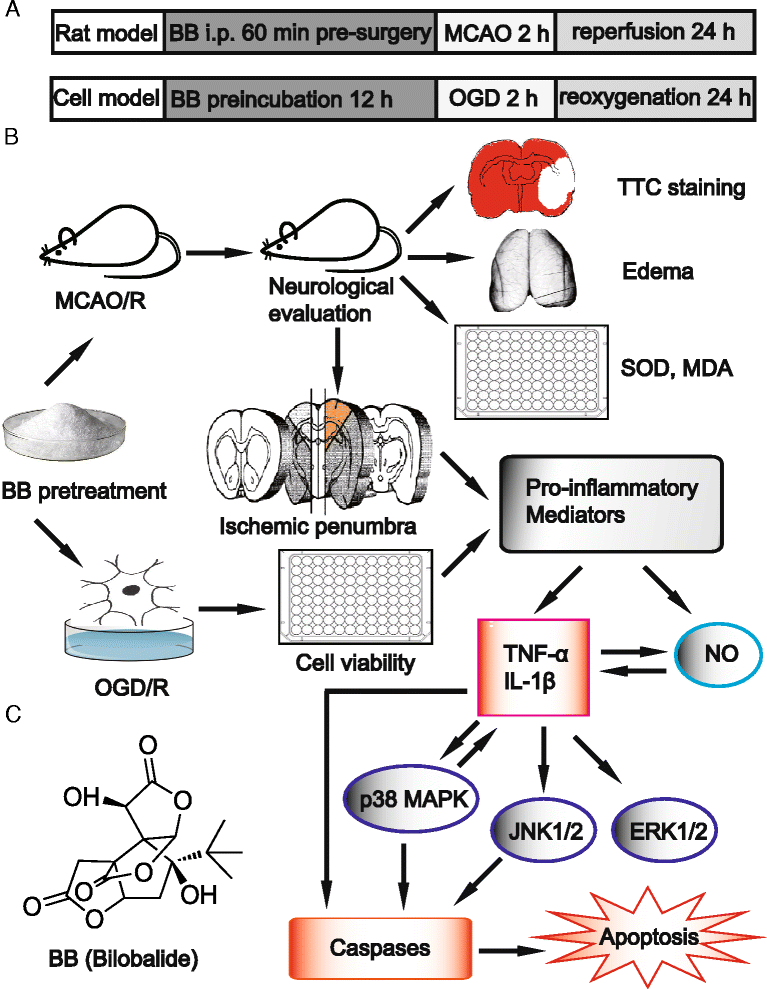
Background: Mitogen-activated protein kinase (MAPK) signaling pathways are implicated in inflammatory and apoptotic processes of cerebral ischemia and reperfusion (I/R) injury. Hence, MAPK pathways represent a promising therapeutic target. Exploring the full potential of inhibitors of MAPK pathways is a useful therapeutic strategy for ischemic stroke. Bilobalide, a predominant sesquiterpene trilactone constituent of Ginkgo biloba leaves, has been shown to exert powerful neuroprotective properties, which are closely related to both anti-inflammatory and anti-apoptotic pathways. We investigated the neuroprotective roles of bilobalide in the models of middle cerebral artery occlusion and reperfusion (MCAO/R) and oxygen-glucose deprivation and reoxygenation (OGD/R) of cerebral I/R injury. Moreover, we attempted to confirm the hypothesis that its protection effect is via modulation of pro-inflammatory mediators and MAPK pathways.
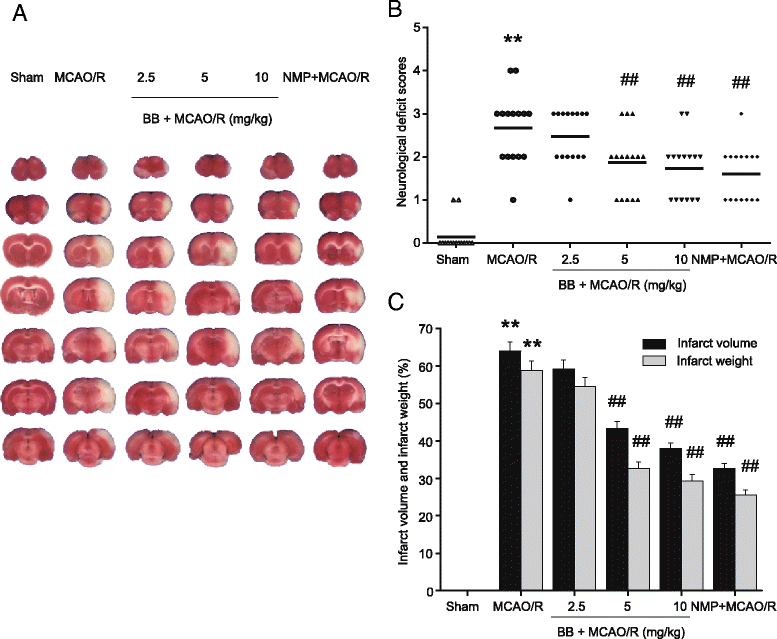
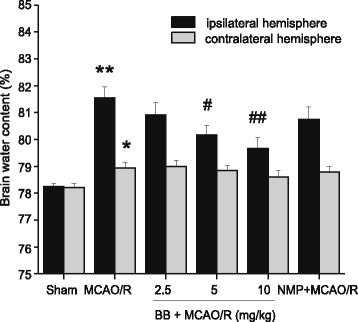
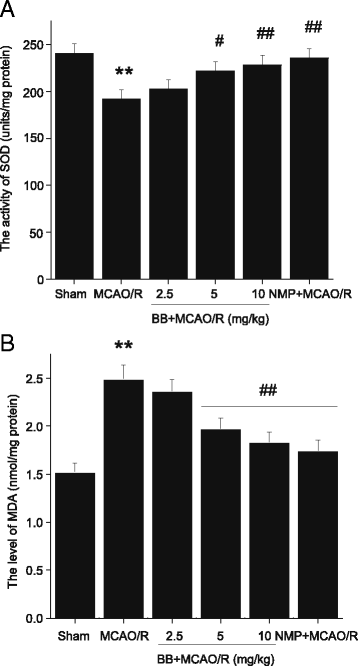
Methods: Male Sprague-Dawley rats were subjected to MCAO for 2 h followed by reperfusion for 24 h. Bilobalide was administered intraperitoneally 60 min before induction of middle cerebral artery occlusion (MCAO). After reperfusion, neurological deficit scores, infarct volume, infarct weight, and brain edema were assessed. Ischemic penumbrae of the cerebral cortex were harvested to determine superoxide dismutase (SOD), malondialdehyde (MDA), nitric oxide, TNF-α, interleukin 1β (IL-1β), p-ERK1/2, p-JNK1/2, and p-p38 MAPK concentration. Similarly, the influence of bilobalide on the expression of nitric oxide, TNF-α, IL-1β, p-ERK1/2, p-JNK1/2, and p-p38 MAPK was also observed in an OGD/R in vitro model of I/R injury.
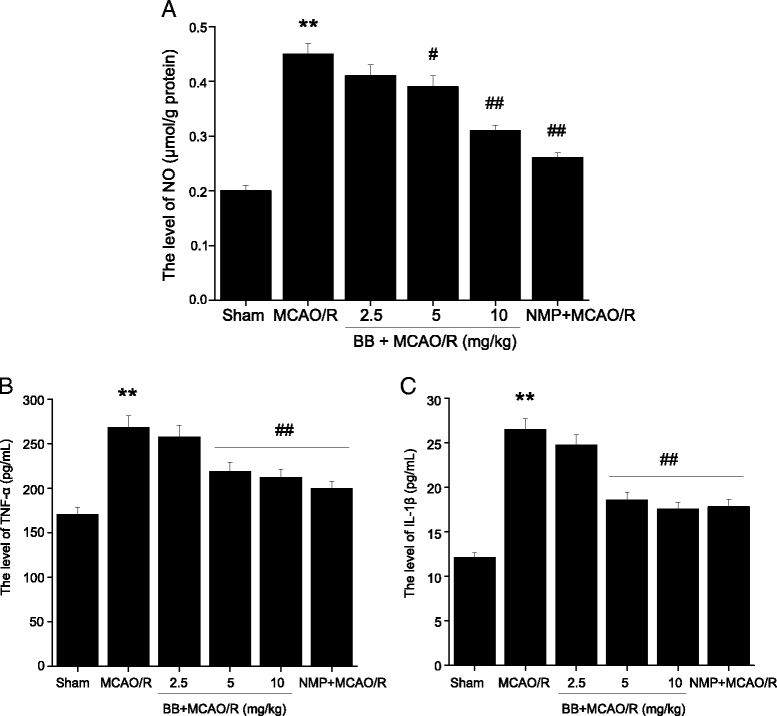
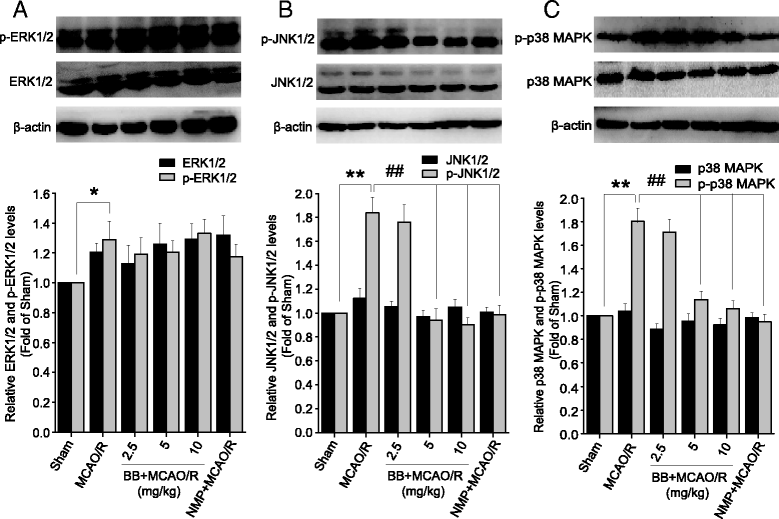
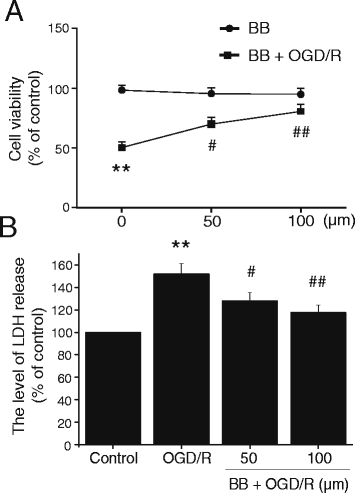
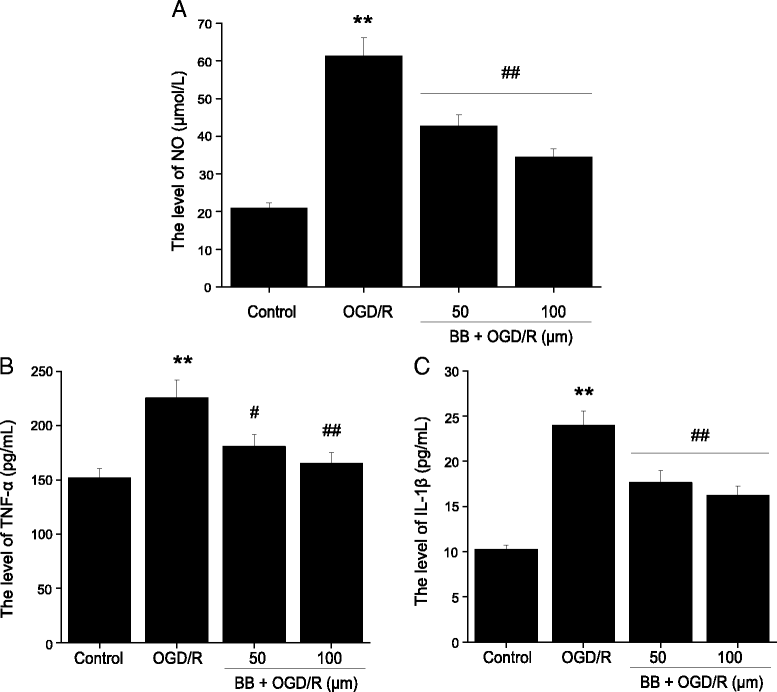
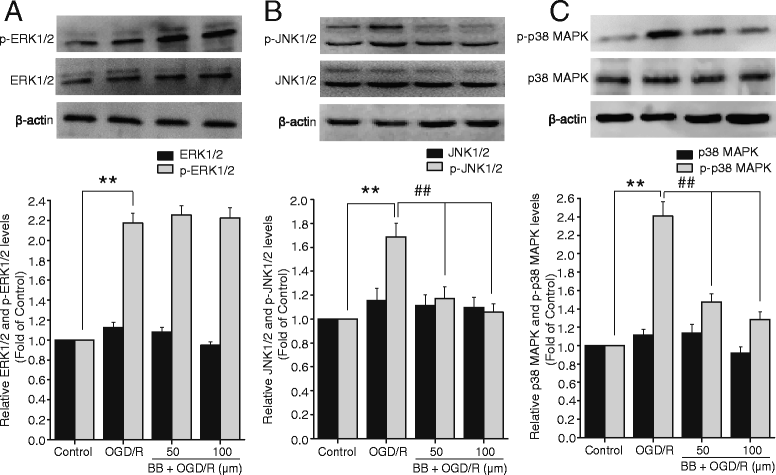
Results: Pretreatment with bilobalide (5, 10 mg/kg) significantly decreased neurological deficit scores, infarct volume, infarct weight, brain edema, and concentrations of MDA, nitric oxide, TNF-α, IL-1β, and increased SOD activity. Furthermore, bilobalide (5, 10 mg/kg) pretreatment significantly down-regulated both p-JNK1/2 and p-p38 MAPK expression, whereas they had no effect on p-ERK1/2 expression in the ischemic penumbra. Supporting these observations in vivo, pretreatment with bilobalide (50, 100 μM) significantly down-regulated nitric oxide, TNF-α, IL-1β, p-JNK1/2, and p-p38 MAPK expression, but did not change p-ERK1/2 expression in rat cortical neurons after OGD/R injury.
Conclusions: These data indicate that the neuroprotective effects of bilobalide on cerebral I/R injury are associated with its inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation.
Figures
References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, et al. Heart disease and stroke statistics - 2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. - DOI - PMC - PubMed
-
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H, American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

