The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: a systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012
- PMID: 25256890
- PMCID: PMC4192762
- DOI: 10.1186/1472-6882-14-362
The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: a systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012
Abstract
Background: The Consolidated Standards for Reporting Trials (CONSORT) are aimed to standardize clinical trial reporting. Our objective is to compare the quality of randomized clinical trials (RCTs) of traditional Chinese medicine (TCM) published in 2005-2009 and 2011-2012 according to the current CONSORT statements and Jadad scale.
Data sources: Reports on RCTs of TCM in the China National Knowledge Infrastructure database (CNKI database) for manuscripts published from 2005 to 2009 and 2011-2012. Search terms included TCM and clinical trial.
Study selection: Manuscripts that reported RCTs of TCM were included.
Data extraction: Independent extraction of articles was done by 3 authors. Disagreement was discussed until agreement was reached. According to the CONSORT checklist, an item was scored as 1 when the item was described in the paper. Otherwise the item was scored as 0.
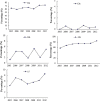
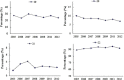
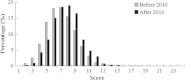
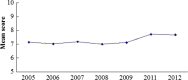
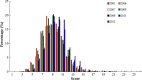
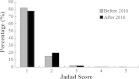
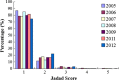
Results: A total of 4133 trials in 2005-2009 and 2861 trials in 2011-2012 were identified respectively. There was a significant increase in proportion of reports that included details of background (24.71% vs 35.20%, P < 0.001), participants (49.79% vs 65.26%, P < 0.001), the methods of random sequence generation (13.77% vs 19.85%, P < 0.001), statistical methods (63.00% vs 72.77%, P < 0.001) and recruitment date (70.14% vs 80.36%, P < 0.001) in 2011-2012 compared to 2005-2009. However, the percentage of reports with trial design decreased from 4.45% to 3.25% (P = 0.011). Few reports described the blinding methods, and there was a decreasing tendency (4.77% vs 2.48%, P < 0.001). There was a similar decreasing tendency on the reporting of funding (6.53% vs 5.00%, P = 0.007). There were no significant differences in the other CONSORT items. In terms of Jadad Score, the proportion of reports with a score of 2 was markedly increased (15.15% vs 19.71%, P < 0.001).
Conclusions: Although the quality of reporting RCTs of TCM was improved in 2011-2012 compared to 2005-2009, the percentages of high-quality reports are both very low in terms of Jadad score. There is a need for improving standards for reporting RCTs in China.
Figures
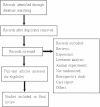
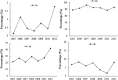
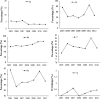
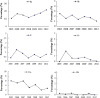
Similar articles
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article.
-
Chinese herbal medicine for oesophageal cancer.Cochrane Database Syst Rev. 2016 Jan 22;2016(1):CD004520. doi: 10.1002/14651858.CD004520.pub7. Cochrane Database Syst Rev. 2016. PMID: 26799001 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Assessment of the Reporting Quality of Placebo-controlled Randomized Trials on the Treatment of Type 2 Diabetes With Traditional Chinese Medicine in Mainland China: A PRISMA-Compliant Systematic Review.Medicine (Baltimore). 2016 Jan;95(3):e2522. doi: 10.1097/MD.0000000000002522. Medicine (Baltimore). 2016. PMID: 26817893 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Observational studies of traditional Chinese medicine may provide evidence nearly consistent with the randomized controlled trials: A meta-epidemiological study.Integr Med Res. 2022 Dec;11(4):100889. doi: 10.1016/j.imr.2022.100889. Epub 2022 Oct 9. Integr Med Res. 2022. PMID: 36345486 Free PMC article.
-
The use of complementary and integrative therapies as adjunct interventions during radiotherapy: a systematic review.Support Care Cancer. 2021 Nov;29(11):6201-6209. doi: 10.1007/s00520-021-06173-1. Epub 2021 Apr 6. Support Care Cancer. 2021. PMID: 33822240
-
Efficacy of Human Papillomavirus L1 Protein Vaccines (Cervarix and Gardasil) in Reducing the Risk of Cervical Intraepithelial Neoplasia: A Meta-analysis.Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16. eCollection 2017. Int J Prev Med. 2017. PMID: 28656100 Free PMC article. Review.
-
Does the medical literature remain inadequately described despite having reporting guidelines for 21 years? - A systematic review of reviews: an update.J Multidiscip Healthc. 2018 Sep 27;11:495-510. doi: 10.2147/JMDH.S155103. eCollection 2018. J Multidiscip Healthc. 2018. PMID: 30310289 Free PMC article. Review.
-
The Therapeutic Principle of Combined Strengthening Qi and Eliminating Pathogens in Treating Middle-Advanced Primary Liver Cancer: A Systematic Review and Meta-Analysis.Front Pharmacol. 2021 Oct 27;12:714287. doi: 10.3389/fphar.2021.714287. eCollection 2021. Front Pharmacol. 2021. PMID: 34776950 Free PMC article. Review.
References
-
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, Decullier E, Easterbrook PJ, Von Elm E, Gamble C, Ghersi D, Ioannidis JP, Simes J, Williamson PR. Systematic review of the empirical evidence of study publication bias and out-come reporting bias. PLoS One. 2008;3:e3081. doi: 10.1371/journal.pone.0003081. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/362/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

