The riluzole derivative 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a mixed KCa2 activator and NaV blocker, is a potent novel anticonvulsant
- PMID: 25256961
- PMCID: PMC4322077
- DOI: 10.1007/s13311-014-0305-y
The riluzole derivative 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a mixed KCa2 activator and NaV blocker, is a potent novel anticonvulsant
Abstract
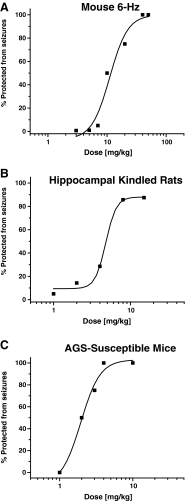
Inhibitors of voltage-gated sodium channels (Na(v)) have been used as anticonvulsants since the 1940s, while potassium channel activators have only been investigated more recently. We here describe the discovery of 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a thioanalog of riluzole, as a potent, novel anticonvulsant, which combines the two mechanisms. SKA-19 is a use-dependent NaV channel blocker and an activator of small-conductance Ca(2+)-activated K(+) channels. SKA-19 reduces action potential firing and increases medium afterhyperpolarization in CA1 pyramidal neurons in hippocampal slices. SKA-19 is orally bioavailable and shows activity in a broad range of rodent seizure models. SKA-19 protects against maximal electroshock-induced seizures in both rats (ED50 1.6 mg/kg i.p.; 2.3 mg/kg p.o.) and mice (ED50 4.3 mg/kg p.o.), and is also effective in the 6-Hz model in mice (ED50 12.2 mg/kg), Frings audiogenic seizure-susceptible mice (ED50 2.2 mg/kg), and the hippocampal kindled rat model of complex partial seizures (ED50 5.5 mg/kg). Toxicity tests for abnormal neurological status revealed a therapeutic index (TD50/ED50) of 6-9 following intraperitoneal and of 33 following oral administration. SKA-19 further reduced acute pain in the formalin pain model and raised allodynic threshold in a sciatic nerve ligation model. The anticonvulsant profile of SKA-19 is comparable to riluzole, which similarly affects Na(V) and KCa2 channels, except that SKA-19 has a ~4-fold greater duration of action owing to more prolonged brain levels. Based on these findings we propose that compounds combining KCa2 channel-activating and Na(v) channel-blocking activity exert broad-spectrum anticonvulsant and analgesic effects.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

