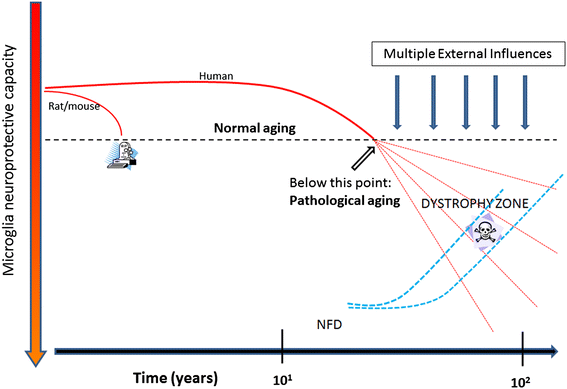
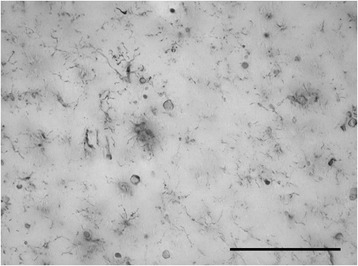
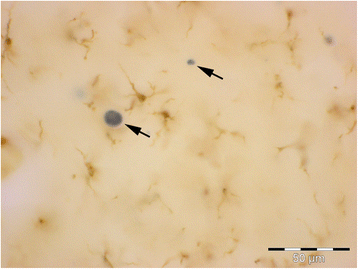
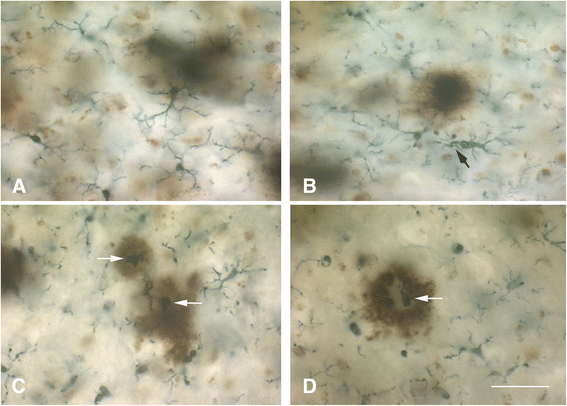
Microglial pathology
- PMID: 25257319
- PMCID: PMC4180960
- DOI: 10.1186/s40478-014-0142-6
Microglial pathology
Abstract
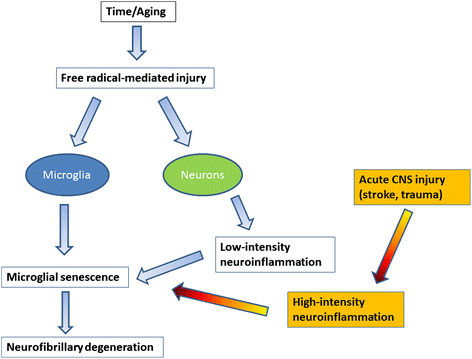
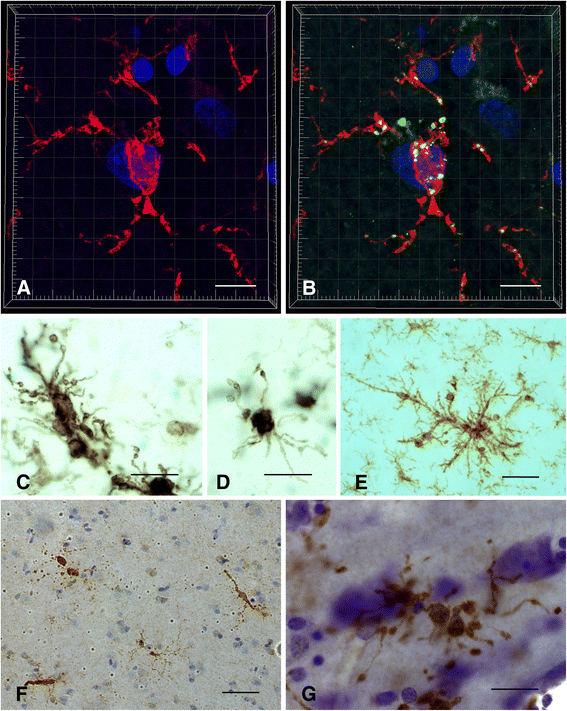
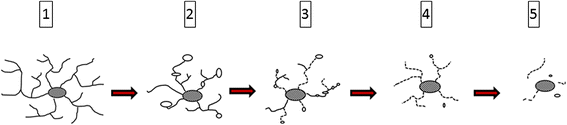
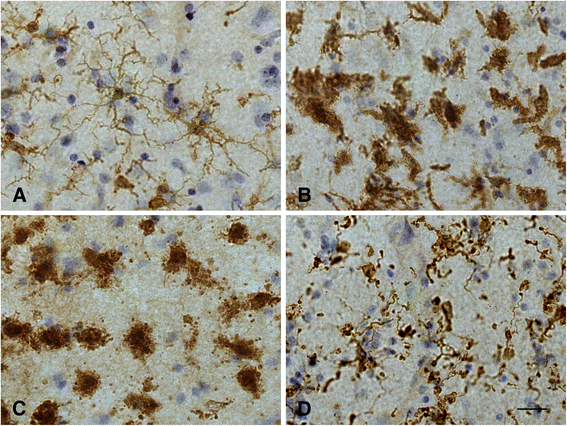
This paper summarizes pathological changes that affect microglial cells in the human brain during aging and in aging-related neurodegenerative diseases, primarily Alzheimer's disease (AD). It also provides examples of microglial changes that have been observed in laboratory animals during aging and in some experimentally induced lesions and disease models. Dissimilarities and similarities between humans and rodents are discussed in an attempt to generate a current understanding of microglial pathology and its significance during aging and in the pathogenesis of Alzheimer dementia (AD). The identification of dystrophic (senescent) microglia has created an ostensible conflict with prior work claiming a role for activated microglia and neuroinflammation during normal aging and in AD, and this has raised a basic question: does the brain's immune system become hyperactive (inflamed) or does it become weakened (senescent) in elderly and demented people, and what is the impact on neuronal function and cognition? Here we strive to reconcile these seemingly contradictory notions by arguing that both low-grade neuroinflammation and microglial senescence are the result of aging-associated free radical injury. Both processes are damaging for microglia as they synergistically exhaust this essential cell population to the point where the brain's immune system is effete and unable to support neuronal function.
Figures

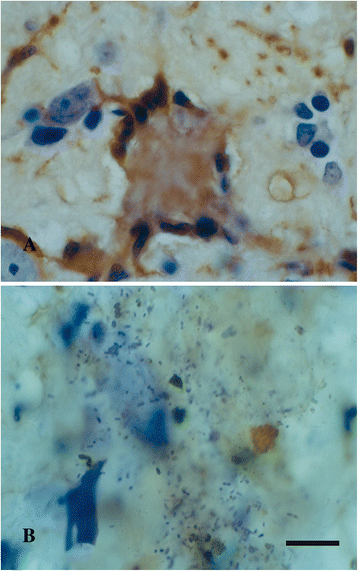
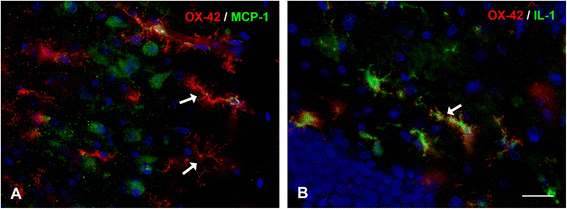
References
-
- v Eitzen U, Egensperger R, Kosel S, Grasbon-Frodl EM, Imai Y, Bise K, Kohsaka S, Mehraein P, Graeber MB. Microglia and the development of spongiform change in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 1998;57(3):246–256. - PubMed
-
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013;126(4):461–477. - PubMed
-
- Biber K, Owens T, Boddeke E. What is microglia neurotoxicity (Not)? Glia. 2014;62(6):841–854. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

