Dynamic mirror-symmetry breaking in bicontinuous cubic phases
- PMID: 25257551
- PMCID: PMC4501316
- DOI: 10.1002/anie.201406907
Dynamic mirror-symmetry breaking in bicontinuous cubic phases
Abstract
Chiral segregation of enantiomers or chiral conformers of achiral molecules during self-assembly in well-ordered crystalline superstructures has fascinated chemists since Pasteur. Here we report spontaneous mirror-symmetry breaking in cubic phases formed by achiral multichain-terminated diphenyl-2,2'-bithiophenes. It was found that stochastic symmetry breaking is a general phenomenon observed in bicontinuous cubic liquid crystal phases of achiral rod-like compounds. In all compounds studied the Im3̄m cubic phase is always chiral, while the Ia3̄d phase is achiral. These intriguing observations are explained by propagation of homochiral helical twist across the entire networks through helix matching at network junctions. In the Ia3̄d phase the opposing chiralities of the two networks cancel, but not so in the three-networks Im3̄m phase. The high twist in the Im3̄m phase explains its previously unrecognized chirality, as well as the origin of this complex structure and the transitions between the different cubic phases.
Keywords: chiral isotropic liquid; conglomerate; deracemization; polycatenar liquid crystal; spontaneous chiral induction.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures

 (“gyroid”) phase; b) the triple-network ${{\it Im}\bar 3m}$
(“gyroid”) phase; b) the triple-network ${{\it Im}\bar 3m}$ phase. Each of the infinite networks is coloured differently. In (b) the red and blue are the identical “inner” and “outer” networks, mutually related by a (1/2 1/2 1/2) translation; yellow is the “middle” network. Equivalent figures with the added minimum surface are shown in SI.
phase. Each of the infinite networks is coloured differently. In (b) the red and blue are the identical “inner” and “outer” networks, mutually related by a (1/2 1/2 1/2) translation; yellow is the “middle” network. Equivalent figures with the added minimum surface are shown in SI.
 phase of compound 1 f at T=112 °C as obtained on cooling from the achiral IsoHT phase, c) IsoLT[*] phase of compound 1 e (T=177 °C) and d) ${{\it Im}\bar 3m}$
phase of compound 1 f at T=112 °C as obtained on cooling from the achiral IsoHT phase, c) IsoLT[*] phase of compound 1 e (T=177 °C) and d) ${{\it Im}\bar 3m}$ phase (T=175 °C) as observed after transition from the IsoLT[*] phase; note that the domain boundaries between the chiral domains are slightly shifted. e,f) Growths of the domains of the ${Ia\bar 3d}$
phase (T=175 °C) as observed after transition from the IsoLT[*] phase; note that the domain boundaries between the chiral domains are slightly shifted. e,f) Growths of the domains of the ${Ia\bar 3d}$ phase at the IsoLT[*]-${Ia\bar 3d}$
phase at the IsoLT[*]-${Ia\bar 3d}$ transition as observed for compound 1 b at T=160 °C (white arrow indicates a seed of the ${Ia\bar 3d}$
transition as observed for compound 1 b at T=160 °C (white arrow indicates a seed of the ${Ia\bar 3d}$ phase); note that during formation of the cubic phase the chirality of the IsoLT[*] phase is completely extinguished (see also videos in SI).
phase); note that during formation of the cubic phase the chirality of the IsoLT[*] phase is completely extinguished (see also videos in SI).
 (120–125 °C) and the IsoHT liquid (130–140 °C). On cooling back to the Cub[*]/${{\it Im}\bar 3m}$
(120–125 °C) and the IsoHT liquid (130–140 °C). On cooling back to the Cub[*]/${{\it Im}\bar 3m}$ (dashed curves) chirality reverses. b) Helical conformations as computed for a model compound related to compounds 1 b,e with OCH3 groups instead of the long alkyloxy chains.[23] c,d) DSC cooling thermograms of c) 1 c and 1 f with direct IsoHT-Cub transitions and d) 1 d[23] and 1 e with an intermediate IsoLT[*] phase (see also Figures S1–S6).
(dashed curves) chirality reverses. b) Helical conformations as computed for a model compound related to compounds 1 b,e with OCH3 groups instead of the long alkyloxy chains.[23] c,d) DSC cooling thermograms of c) 1 c and 1 f with direct IsoHT-Cub transitions and d) 1 d[23] and 1 e with an intermediate IsoLT[*] phase (see also Figures S1–S6).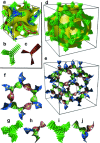
 phase decorated with schematic mesogens (rod-like molecular cores, green) showing the molecular twist along the network segments. The gyroid minimum surface is also shown (yellow) and b,c) show the network junctions. d) The same for the middle of the three networks of the ${{\it Im}\bar 3m}$
phase decorated with schematic mesogens (rod-like molecular cores, green) showing the molecular twist along the network segments. The gyroid minimum surface is also shown (yellow) and b,c) show the network junctions. d) The same for the middle of the three networks of the ${{\it Im}\bar 3m}$ phase (yellow network in Figure 1 b). This network closely follows the Schwartz P-type minimum surface (shown in yellow). e) The middle ${{\it Im}\bar 3m}$
phase (yellow network in Figure 1 b). This network closely follows the Schwartz P-type minimum surface (shown in yellow). e) The middle ${{\it Im}\bar 3m}$ network shown as ribbons containing the molecular axes axis (black rods) and f) loop of 6 junctions in this network. g–j) Details of the two types of junctions in the ${{\it Im}\bar 3m}$
network shown as ribbons containing the molecular axes axis (black rods) and f) loop of 6 junctions in this network. g–j) Details of the two types of junctions in the ${{\it Im}\bar 3m}$ phase in mesogen (g,i) and ribbon (h,j) representations.
phase in mesogen (g,i) and ribbon (h,j) representations.References
-
- Pasteur JL. Ann. Chim. Phys. 1848;24:442–459.
-
- Weissbuch I, Leiserowitz L, Lahav M. Top. Curr. Chem. 2005;259:123–163.
-
- Amabilino DB. Chirality at the Nanoscale. Weinheim: Wiley-VCH; 2009.
-
- Ernst KH. Top. Curr. Chem. 2006;265:209–252.
-
- Reddy RA, Tschierske C. J. Mater. Chem. 2006;16:907–961.
LinkOut - more resources
Full Text Sources
Other Literature Sources

