Trafficking of ThermoTRP Channels
- PMID: 25257900
- PMCID: PMC4194048
- DOI: 10.3390/membranes4030525
Trafficking of ThermoTRP Channels
Abstract
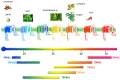
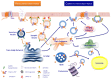
ThermoTRP channels (thermoTRPs) define a subfamily of the transient receptor potential (TRP) channels that are activated by changes in the environmental temperature, from noxious cold to injurious heat. Acting as integrators of several stimuli and signalling pathways, dysfunction of these channels contributes to several pathological states. The surface expression of thermoTRPs is controlled by both, the constitutive and regulated vesicular trafficking. Modulation of receptor surface density during pathological processes is nowadays considered as an interesting therapeutic approach for management of diseases, such as chronic pain, in which an increased trafficking is associated with the pathological state. This review will focus on the recent advances trafficking of the thermoTRP channels, TRPV1, TRPV2, TRPV4, TRPM3, TRPM8 and TRPA1, into/from the plasma membrane. Particularly, regulated membrane insertion of thermoTRPs channels contributes to a fine tuning of final channel activity, and indeed, it has resulted in the development of novel therapeutic approaches with successful clinical results such as disruption of SNARE-dependent exocytosis by botulinum toxin or botulinomimetic peptides.
Figures
References
-
- Flockerzi V., Nilius B. TRPs: Truly Remarkable Proteins. In: Nilius B., Flockerzi V., editors. Mammalian Transient Receptor Potential (TRP) Cation Channels. Volume 222. Handbook of Experimental Pharmacology; Springer; Berlin, Germany: 2014. pp. 1–12.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

