mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity
- PMID: 25258083
- PMCID: PMC4226238
- DOI: 10.1126/science.1250684
mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity
Erratum in
- Science. 2014 Nov 7;346(6210):aaa1503. van der Meer, Brian M J W [corrected to van der Veer, Brian M J W]
Abstract
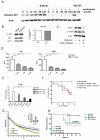
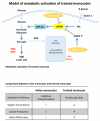
Epigenetic reprogramming of myeloid cells, also known as trained immunity, confers nonspecific protection from secondary infections. Using histone modification profiles of human monocytes trained with the Candida albicans cell wall constituent β-glucan, together with a genome-wide transcriptome, we identified the induced expression of genes involved in glucose metabolism. Trained monocytes display high glucose consumption, high lactate production, and a high ratio of nicotinamide adenine dinucleotide (NAD(+)) to its reduced form (NADH), reflecting a shift in metabolism with an increase in glycolysis dependent on the activation of mammalian target of rapamycin (mTOR) through a dectin-1-Akt-HIF-1α (hypoxia-inducible factor-1α) pathway. Inhibition of Akt, mTOR, or HIF-1α blocked monocyte induction of trained immunity, whereas the adenosine monophosphate-activated protein kinase activator metformin inhibited the innate immune response to fungal infection. Mice with a myeloid cell-specific defect in HIF-1α were unable to mount trained immunity against bacterial sepsis. Our results indicate that induction of aerobic glycolysis through an Akt-mTOR-HIF-1α pathway represents the metabolic basis of trained immunity.
Copyright © 2014, American Association for the Advancement of Science.
Figures



Comment in
-
Macrophages: innate memory training.Nat Rev Immunol. 2014 Nov;14(11):713. doi: 10.1038/nri3759. Epub 2014 Oct 10. Nat Rev Immunol. 2014. PMID: 25301255 No abstract available.
References
-
- Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annual review of plant biology. 2013;64:839. - PubMed
-
- Kurtz J. Specific memory within innate immune systems. Trends in immunology. 2005 Apr;26:186. - PubMed
-
- Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the "adaptive" form of innate immunity. Microbes and infection / Institut Pasteur. 2007 Nov-Dec;9:1680. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

