Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date
- PMID: 25258511
- PMCID: PMC4174046
- DOI: 10.2147/DDDT.S55587
Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date
Abstract
Obesity is an emerging disease worldwide. Changes in living habits, especially with increased consumption of high-calorie foods and decreased levels of physical activity, lead to an energy imbalance that brings weight gain. Overweight and obesity are major risk factors for several chronic diseases (including cardiovascular diseases, diabetes, and cancer), reduce quality of life, and are associated with higher mortality. For all these reasons, it is of the utmost importance that the trend be reversed and obese people enabled to lose weight. It is known that eating a healthy diet and exercising regularly can help prevent obesity, but data show that in many cases these steps are not enough. This is the reason why, over the last few decades, several antiobesity drugs have been developed. However, the disappointing results demonstrated for the vast majority of them have not discouraged the pharmaceutical industry from continuing to look for an effective drug or combination of drugs. The systematic review presented here focuses on naltrexone sustained-release/bupropion sustained-release combination (Contrave(®)). We conclude from the current published reports that its effectiveness in the treatment of obesity can be estimated as a placebo-subtracted weight loss of around 4.5%. This weight reduction is moderate but similar to other antiobesity drugs. The safety profile of this combination is acceptable, despite additional data regarding cardiovascular disease being needed.
Keywords: Contrave; cancer; cardiovascular disease; diabetes; overweight; weight loss.
Figures
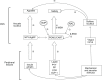
Comment in
-
Letter to the editor: naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date.Drug Des Devel Ther. 2015 Jan 8;9:419-23. doi: 10.2147/DDDT.S77881. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25609921 Free PMC article. No abstract available.
References
-
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. - PubMed
-
- Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. - PubMed
-
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:5w822–5w831. - PubMed
-
- National Heart, Lung and Blood Institute (NHLBI) Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: NHLBI; 1998. [Accessed May 15, 2014]. Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US) (Report number 98-4083). Available from: http://www.ncbi.nlm.nih.gov/books/NBK2003/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

