Photothermal Treatment of Human Pancreatic Cancer Using PEGylated Multi-Walled Carbon Nanotubes Induces Apoptosis by Triggering Mitochondrial Membrane Depolarization Mechanism
- PMID: 25258649
- PMCID: PMC4174512
- DOI: 10.7150/jca.9481
Photothermal Treatment of Human Pancreatic Cancer Using PEGylated Multi-Walled Carbon Nanotubes Induces Apoptosis by Triggering Mitochondrial Membrane Depolarization Mechanism
Abstract
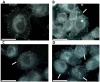
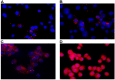
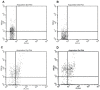
Pancreatic cancer (PC) is one of the most lethal solid tumor in humans, with an overall 5-year survival rate of less than 5%. Thermally active carbon nanotubes have already brought to light promising results in PC research and treatment. We report here the construct of a nano-biosystem based on multi-walled carbon nanotubes and polyethylene glycol (PEG) molecules validated through AFM, UV-Vis and DLS. We next studied the photothermal effect of these PEG-ylated multi-walled carbon nanotubes (5, 10 and 50 μg/mL, respectively) on pancreatic cancer cells (PANC-1) and further analyzed the molecular and cellular events involved in cell death occurrence. Using cell proliferation, apoptosis, membrane polarization and oxidative stress assays for ELISA, fluorescence microscopy and flow cytometry we show here that hyperthermia following MWCNTs-PEG laser mediated treatment (808 nm, 2W) leads to mitochondrial membrane depolarization that activates the flux of free radicals within the cell and the oxidative state mediate cellular damage in PC cells via apoptotic pathway. Our results are of decisive importance especially in regard with the development of novel nano-biosystems capable to target mitochondria and to synergically act both as cytotoxic drug as well as thermally active agents in order to overcome one of the most common problem met in oncology, that of intrinsic resistance to chemotherapeutics.
Keywords: PEG functionalization; apoptosis; carbon nanotubes; mitochondrial therapy.; pancreatic cancer; photothermal ablation.
Conflict of interest statement
Competing Interests: None to declare.
Figures



References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Mocan L, Ilie I, Tabaran FA, Dana B, Zaharie F, Zdrehus C, Puia C. et al. Surface plasmon resonance-induced photoactivation of gold nanoparticles as mitochondria-targeted therapeutic agents for pancreatic cancer. Expert opinion on therapeutic targets. 2013;(0):1–11. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

