Melatonin Reverses Fas, E2F-1 and Endoplasmic Reticulum Stress Mediated Apoptosis and Dysregulation of Autophagy Induced by the Herbicide Atrazine in Murine Splenocytes
- PMID: 25259610
- PMCID: PMC4178181
- DOI: 10.1371/journal.pone.0108602
Melatonin Reverses Fas, E2F-1 and Endoplasmic Reticulum Stress Mediated Apoptosis and Dysregulation of Autophagy Induced by the Herbicide Atrazine in Murine Splenocytes
Abstract
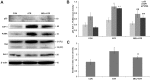
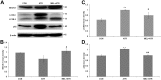
Exposure to the herbicide Atrazine (ATR) can cause immunotoxicity, apart from other adverse consequences for animal and human health. We aimed at elucidating the apoptotic mechanisms involved in immunotoxicity of ATR and their attenuation by Melatonin (MEL). Young Swiss mice were divided into control, ATR and MEL+ATR groups based on daily (x14) intraperitoneal administration of the vehicle (normal saline), ATR (100 mg/kg body weight) and MEL (20 mg/kg body weight) with ATR. Isolated splenocytes were processed for detection of apoptosis by Annexin V-FITC and TUNEL assays, and endoplasmic reticulum (ER) stress by immunostaining. Key proteins involved in apoptosis, ER stress and autophagy were quantified by immunoblotting. ATR treatment resulted in Fas-mediated activation of caspases 8 and 3 and inactivation of PARP1 which were inhibited significantly by co-treatment with MEL. MEL also attenuated the ATR-induced, p53 independent mitochondrial apoptosis through upregulation of E2F-1 and PUMA and suppression of their downstream target Bax. An excessive ER stress triggered by ATR through overexpression of ATF-6α, spliced XBP-1, CREB-2 and GADD153 signals was reversed by MEL. MEL also reversed the ATR-induced impairment of autophagy which was indicated by a decline in BECN-1, along with significant enhancement in LC3B-II and p62 expressions. Induction of mitochondrial apoptosis, ER stress and autophagy dysregulation provide a new insight into the mechanism of ATR immunotoxicity. The cytoprotective role of MEL, on the other hand, was defined by attenuation of ER stress, Fas-mediated and p53 independent mitochondria-mediated apoptosis as well as autophagy signals.
Conflict of interest statement
Figures




References
-
- Rowe AM, Brundage KM, Barnett JB (2008) Developmental immunotoxicity of atrazine in rodents. Basic Clin Pharmacol Toxicol 102: 139–145. - PubMed
-
- Cragin LA, Kesner JS, Bachand AM, Barr DB, Meadows JW, et al. (2011) Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ Res 111: 1293–1301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

