Encapsulating non-human primate multipotent stromal cells in alginate via high voltage for cell-based therapies and cryopreservation
- PMID: 25259731
- PMCID: PMC4178041
- DOI: 10.1371/journal.pone.0107911
Encapsulating non-human primate multipotent stromal cells in alginate via high voltage for cell-based therapies and cryopreservation
Abstract
Alginate cell-based therapy requires further development focused on clinical application. To assess engraftment, risk of mutations and therapeutic benefit studies should be performed in an appropriate non-human primate model, such as the common marmoset (Callithrix jacchus). In this work we encapsulated amnion derived multipotent stromal cells (MSCs) from Callithrix jacchus in defined size alginate beads using a high voltage technique. Our results indicate that i) alginate-cell mixing procedure and cell concentration do not affect the diameter of alginate beads, ii) encapsulation of high cell numbers (up to 10×106 cells/ml) can be performed in alginate beads utilizing high voltage and iii) high voltage (15-30 kV) does not alter the viability, proliferation and differentiation capacity of MSCs post-encapsulation compared with alginate encapsulated cells produced by the traditional air-flow method. The consistent results were obtained over the period of 7 days of encapsulated MSCs culture and after cryopreservation utilizing a slow cooling procedure (1 K/min). The results of this work show that high voltage encapsulation can further be maximized to develop cell-based therapies with alginate beads in a non-human primate model towards human application.
Conflict of interest statement
Figures
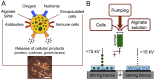

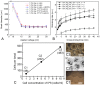
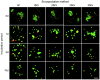



References
-
- Portero A, Orive G, Hernandez RM, Pedraz JL (2010) Cell encapsulation for the treatment of central nervous system disorders. Rev Neurol 50: 409–419. - PubMed
-
- Lee MK, Bae YH (2000) Cell transplantation for endocrine disorders. Adv Drug Deliv Rev 42: 103–120. - PubMed
-
- Wollert KC, Drexler H (2006) Cell-based therapy for heart failure. Curr Opin Cardiol 21: 234–239. - PubMed
-
- Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, et al. (2003) Cell encapsulation: promise and progress. Nat Med 9: 104–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

