Direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients
- PMID: 25259739
- PMCID: PMC4178151
- DOI: 10.1371/journal.pone.0108327
Direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients
Erratum in
-
Correction: Direct Proof of the In Vivo Pathogenic Role of the AChR Autoantibodies from Myasthenia Gravis Patients.PLoS One. 2015 Jan 30;10(1):e0117673. doi: 10.1371/journal.pone.0117673. eCollection 2015. PLoS One. 2015. PMID: 25635870 Free PMC article. No abstract available.
-
Correction: direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients.PLoS One. 2015 Mar 25;10(3):e0120947. doi: 10.1371/journal.pone.0120947. eCollection 2015. PLoS One. 2015. PMID: 25807480 Free PMC article. No abstract available.
Abstract
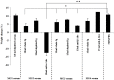
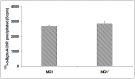
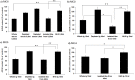
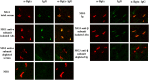
Several studies have suggested that the autoantibodies (autoAbs) against muscle acetylcholine receptor (AChR) of myasthenia gravis (MG) patients are the main pathogenic factor in MG; however, this belief has not yet been confirmed with direct observations. Although animals immunized with AChR or injected with anti-AChR monoclonal Abs, or with crude human MG Ig fractions exhibit MG symptoms, the pathogenic role of isolated anti-AChR autoAbs, and, more importantly, the absence of pathogenic factor(s) in the autoAb-depleted MG sera has not yet been shown by in vivo studies. Using recombinant extracellular domains of the human AChR α and β subunits, we have isolated autoAbs from the sera of four MG patients. The ability of these isolated anti-subunit Abs and of the Ab-depleted sera to passively transfer experimental autoimmune MG in Lewis rats was investigated. We found that the isolated anti-subunit Abs were at least as efficient as the corresponding whole sera or whole Ig in causing experimental MG. Abs to both α- and β-subunit were pathogenic although the anti-α-subunit were much more efficient than the anti-β-subunit ones. Interestingly, the autoAb-depleted sera were free of pathogenic activity. The later suggests that the myasthenogenic potency of the studied anti-AChR MG sera is totally due to their anti-AChR autoAbs, and therefore selective elimination of the anti-AChR autoAbs from MG patients may be an efficient therapy for MG.
Conflict of interest statement
Figures

References
-
- Lindstrom JM (2000) Acetylcholine receptors and myasthenia. Muscle Nerve 23: 453–477. - PubMed
-
- Vincent A, Palace J, Hilton-Jones D (2001) Myasthenia gravis. Lancet 357: 2122–2128. - PubMed
-
- Bello-Rivero I, Cervantes M, Torres Y, Ferrero J, Rodríguez E, et al. (2004) Characterization of the immunoreactivity of anti-interferon alpha antibodies in myasthenia gravis patients. Epitope mapping. J Autoimmun 23: 63–73. - PubMed
-
- Infante AJ, Baillargeon J, Kraig E, Lott L, Jackson C, et al. (2003) Evidence of a diverse T cell receptor repertoire for acetylcholine receptor, the autoantigen of myasthenia gravis. J Autoimmun 21: 167–174. - PubMed
-
- Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B (2014) Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun 48–49: 143–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

