Oligoclonal CD8+ T cells play a critical role in the development of hypertension
- PMID: 25259750
- PMCID: PMC4191997
- DOI: 10.1161/HYPERTENSIONAHA.114.04147
Oligoclonal CD8+ T cells play a critical role in the development of hypertension
Abstract
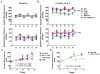
Recent studies have emphasized a role of adaptive immunity, and particularly T cells, in the genesis of hypertension. We sought to determine the T-cell subtypes that contribute to hypertension and renal inflammation in angiotensin II-induced hypertension. Using T-cell receptor spectratyping to examine T-cell receptor usage, we demonstrated that CD8(+) cells, but not CD4(+) cells, in the kidney exhibited altered T-cell receptor transcript lengths in Vβ3, 8.1, and 17 families in response to angiotensin II-induced hypertension. Clonality was not observed in other organs. The hypertension caused by angiotensin II in CD4(-/-) and MHCII(-/-) mice was similar to that observed in wild-type mice, whereas CD8(-/-) mice and OT1xRAG-1(-/-) mice, which have only 1 T-cell receptor, exhibited a blunted hypertensive response to angiotensin II. Adoptive transfer of pan T cells and CD8(+) T cells but not CD4(+)/CD25(-) cells conferred hypertension to RAG-1(-/-) mice. In contrast, transfer of CD4(+)/CD25(+) cells to wild-type mice receiving angiotensin II decreased blood pressure. Mice treated with angiotensin II exhibited increased numbers of kidney CD4(+) and CD8(+) T cells. In response to a sodium/volume challenge, wild-type and CD4(-/-) mice infused with angiotensin II retained water and sodium, whereas CD8(-/-) mice did not. CD8(-/-) mice were also protected against angiotensin-induced endothelial dysfunction and vascular remodeling in the kidney. These data suggest that in the development of hypertension, an oligoclonal population of CD8(+) cells accumulates in the kidney and likely contributes to hypertension by contributing to sodium and volume retention and vascular rarefaction.
Keywords: adaptive immunity; angiotensin II; diuresis; natriuresis; receptors, antigen, T-cell.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures




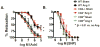
References
-
- Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodriguez-Artalejo F, Stegmayr B, Thamm M, Tuomilehto J, Vanuzzo D, Vescio F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. - PubMed
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL039006/HL/NHLBI NIH HHS/United States
- P01HL095070/HL/NHLBI NIH HHS/United States
- R01 HL108701/HL/NHLBI NIH HHS/United States
- R01HL10529402/HL/NHLBI NIH HHS/United States
- P01 HL095070/HL/NHLBI NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- F32 HL124972/HL/NHLBI NIH HHS/United States
- T32 HL069765/HL/NHLBI NIH HHS/United States
- R01 HL105294/HL/NHLBI NIH HHS/United States
- R01HL039006/HL/NHLBI NIH HHS/United States
- P01GM015431/GM/NIGMS NIH HHS/United States
- T32GM07569/GM/NIGMS NIH HHS/United States
- P01HL058000/HL/NHLBI NIH HHS/United States
- T32 GM007569/GM/NIGMS NIH HHS/United States
- R01 HL002334/HL/NHLBI NIH HHS/United States
- K08 HL121671/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

