Recombinant p35 from bacteria can form Interleukin (IL-)12, but Not IL-35
- PMID: 25259790
- PMCID: PMC4178060
- DOI: 10.1371/journal.pone.0107990
Recombinant p35 from bacteria can form Interleukin (IL-)12, but Not IL-35
Abstract
The Interleukin (IL)-12 family contains several heterodimeric composite cytokines which share subunits among each other. IL-12 consists of the subunits p40 (shared with IL-23) and p35. p35 is shared with the composite cytokine IL-35 which comprises of the p35/EBI3 heterodimer (EBI3 shared with IL-27). IL-35 signals via homo- or heterodimers of IL-12Rβ2, gp130 and WSX-1, which are shared with IL-12 and IL-27 receptor complexes, respectively. p35 was efficiently secreted in complex with p40 as IL-12 but not with EBI3 as IL-35 in several transfected cell lines tested which complicates the analysis of IL-35 signal transduction. p35 and p40 but not p35 and EBI3 form an inter-chain disulfide bridge. Mutation of the responsible cysteine residue (p40C197A) reduced IL-12 formation and activity only slightly. Importantly, the p40C197A mutation prevented the formation of antagonistic p40 homodimers which enabled the in vitro reconstitution of biologically active IL-12 with p35 produced in bacteria (p35bac). Reconstitution of IL-35 with p35bac and EBI3 did, however, fail to induce signal transduction in Ba/F3 cells expressing IL-12Rβ2 and gp130. In summary, we describe the in vitro reconstitution of IL-12, but fail to produce recombinant IL-35 by this novel approach.
Conflict of interest statement
Figures

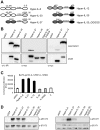


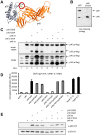



References
-
- Garbers C, Hermanns H, Schaper F, Müller-Newen G, Grötzinger J, et al. (2012) Plasticity and cross-talk of Interleukin 6-type cytokines. Cytokine Growth Factor Rev 23: 85–182. - PubMed
-
- Hölscher C (2004) The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med Microbiol Immunol 193: 1–17. - PubMed
-
- Gillessen S, Carvajal D, Ling P, Podlaski F, Stremlo D, et al. (1995) Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol 25: 200–206. - PubMed
-
- Ling P, Gately M, Gubler U, Stern A, Lin P, et al. (1995) Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol 154: 116–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

