Synthetic CpG islands reveal DNA sequence determinants of chromatin structure
- PMID: 25259796
- PMCID: PMC4204011
- DOI: 10.7554/eLife.03397
Synthetic CpG islands reveal DNA sequence determinants of chromatin structure
Abstract
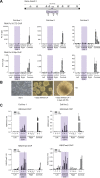
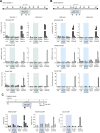
The mammalian genome is punctuated by CpG islands (CGIs), which differ sharply from the bulk genome by being rich in G + C and the dinucleotide CpG. CGIs often include transcription initiation sites and display 'active' histone marks, notably histone H3 lysine 4 methylation. In embryonic stem cells (ESCs) some CGIs adopt a 'bivalent' chromatin state bearing simultaneous 'active' and 'inactive' chromatin marks. To determine whether CGI chromatin is developmentally programmed at specific genes or is imposed by shared features of CGI DNA, we integrated artificial CGI-like DNA sequences into the ESC genome. We found that bivalency is the default chromatin structure for CpG-rich, G + C-rich DNA. A high CpG density alone is not sufficient for this effect, as A + T-rich sequence settings invariably provoke de novo DNA methylation leading to loss of CGI signature chromatin. We conclude that both CpG-richness and G + C-richness are required for induction of signature chromatin structures at CGIs.
Keywords: CpG islands; DNA methylation; bivalent chromatin; chromosomes; genes; histone modifications; mouse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








Comment in
-
How to build your own island.Elife. 2014 Oct 21;3:e04779. doi: 10.7554/eLife.04779. Elife. 2014. PMID: 25333621 Free PMC article.
References
-
- Allen MD, Grummitt CG, Hilcenko C, Min SY, Tonkin LM, Johnson CM, Freund SM, Bycroft M, Warren AJ. 2006. Solution structure of the nonmethyl-CpG-binding CXXC domain of the leukaemia-associated MLL histone methyltransferase. The EMBO Journal 25:4503–4512. doi: 10.1038/sj.emboj.7601340 - DOI - PMC - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326. doi: 10.1016/j.cell.2006.02.041 - DOI - PubMed
-
- Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LL, Ito S, Cooper S, Kondo K, Koseki Y, Ishikura T, Long HK, Sheahan TW, Brockdorff N, Kessler BM, Koseki H, Klose RJ. 2014. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157:1445–1459. doi: 10.1016/j.cell.2014.05.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

