The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells
- PMID: 25259923
- PMCID: PMC4371485
- DOI: 10.1016/j.cell.2014.08.026
The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells
Abstract
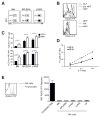
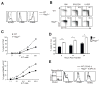
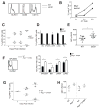
The emergence of recombination-activating genes (RAGs) in jawed vertebrates endowed adaptive immune cells with the ability to assemble a diverse set of antigen receptor genes. In contrast, innate lymphocytes, such as natural killer (NK) cells, are not believed to require RAGs. Here, we report that NK cells unable to express RAGs or RAG endonuclease activity during ontogeny exhibit a cell-intrinsic hyperresponsiveness but a diminished capacity to survive following virus-driven proliferation, a reduced expression of DNA damage response mediators, and defects in the repair of DNA breaks. Evidence for this novel function of RAG has also been observed in T cells and innate lymphoid cells (ILCs), revealing an unexpected role for RAG proteins beyond V(D)J recombination. We propose that DNA cleavage events mediated by RAG endow developing adaptive and innate lymphocytes with a cellular "fitness" that safeguards their persistence later in life during episodes of rapid proliferation or cellular stress.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Natural killer cells: RAG keeps natural killers fit.Nat Rev Immunol. 2014 Nov;14(11):716-7. doi: 10.1038/nri3760. Epub 2014 Oct 10. Nat Rev Immunol. 2014. PMID: 25301254 No abstract available.
-
DNA damage responses: beyond double-strand break repair.Curr Biol. 2015 Jan 5;25(1):R45-6. doi: 10.1016/j.cub.2014.11.024. Curr Biol. 2015. PMID: 25562302
References
-
- Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. - PubMed
-
- Agrawal A, Schatz DG. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. - PubMed
-
- Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88:107–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

