A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases
- PMID: 25259927
- PMCID: PMC4298142
- DOI: 10.1016/j.cell.2014.09.002
A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases
Abstract
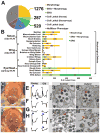
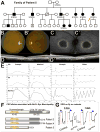
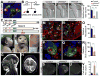
Invertebrate model systems are powerful tools for studying human disease owing to their genetic tractability and ease of screening. We conducted a mosaic genetic screen of lethal mutations on the Drosophila X chromosome to identify genes required for the development, function, and maintenance of the nervous system. We identified 165 genes, most of whose function has not been studied in vivo. In parallel, we investigated rare variant alleles in 1,929 human exomes from families with unsolved Mendelian disease. Genes that are essential in flies and have multiple human homologs were found to be likely to be associated with human diseases. Merging the human data sets with the fly genes allowed us to identify disease-associated mutations in six families and to provide insights into microcephaly associated with brain dysgenesis. This bidirectional synergism between fly genetics and human genomics facilitates the functional annotation of evolutionarily conserved genes involved in human health.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Flies give wings to human disease studies.Nat Methods. 2014 Dec;11(12):1197. doi: 10.1038/nmeth.3200. Nat Methods. 2014. PMID: 25584381 No abstract available.
References
-
- Asencio C, Davidson IF, Santarella-Mellwig R, Ly-Hartig TB, Mall M, Wallenfang MR, Mattaj IW, Gorjanacz M. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell. 2012;150:122–135. - PubMed
-
- Bayat V, Thiffault I, Jaiswal M, Tetreault M, Donti T, Sasarman F, Bernard G, Demers-Lamarche J, Dicaire MJ, Mathieu J, et al. Mutations in the mitochondrial methionyl-tRNA synthetase cause a neurodegenerative phenotype in flies and a recessive ataxia (ARSAL) in humans. PLoS Biol. 2012;10:e1001288. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EY018213/EY/NEI NIH HHS/United States
- U54 HG006542/HG/NHGRI NIH HHS/United States
- U54HG006542/HG/NHGRI NIH HHS/United States
- 5R01GM067858/GM/NIGMS NIH HHS/United States
- R24 EY019861/EY/NEI NIH HHS/United States
- EY019861/EY/NEI NIH HHS/United States
- R01 NS058529/NS/NINDS NIH HHS/United States
- R01 HG011795/HG/NHGRI NIH HHS/United States
- T32 EY013933/EY/NEI NIH HHS/United States
- K08 NS076547/NS/NINDS NIH HHS/United States
- K08NS076547/NS/NINDS NIH HHS/United States
- K12 GM084897/GM/NIGMS NIH HHS/United States
- RC4 GM096355/GM/NIGMS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- P30 EY019007/EY/NEI NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- EY019007/EY/NEI NIH HHS/United States
- K23 NS078056/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 5K12GM084897/GM/NIGMS NIH HHS/United States
- T32 NS043124/NS/NINDS NIH HHS/United States
- EY021163/EY/NEI NIH HHS/United States
- R01 EY021163/EY/NEI NIH HHS/United States
- 1RC4GM096355-01/GM/NIGMS NIH HHS/United States
- T32 NS043124-11/NS/NINDS NIH HHS/United States
- R01 GM067858/GM/NIGMS NIH HHS/United States
- K23NS078056/NS/NINDS NIH HHS/United States
- 5P30HD024064/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

