Structural and functional profiling of environmental ligands for estrogen receptors
- PMID: 25260197
- PMCID: PMC4256047
- DOI: 10.1289/ehp.1408453
Structural and functional profiling of environmental ligands for estrogen receptors
Abstract
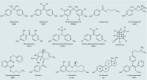
Background: Individuals are exposed daily to environmental pollutants that may act as endocrine-disrupting chemicals (EDCs), causing a range of developmental, reproductive, metabolic, or neoplastic diseases. With their mostly hydrophobic pocket that serves as a docking site for endogenous and exogenous ligands, nuclear receptors (NRs) can be primary targets of small molecule environmental contaminants. However, most of these compounds are chemically unrelated to natural hormones, so their binding modes and associated hormonal activities are hardly predictable.
Objectives: We conducted a correlative analysis of structural and functional data to gain insight into the mechanisms by which 12 members of representative families of pollutants bind to and activate the estrogen receptors ERα and ERβ.
Methods: We used a battery of biochemical, structural, biophysical, and cell-based approaches to characterize the interaction between ERs and their environmental ligands.
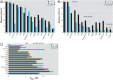
Results: Our study revealed that the chemically diverse compounds bound to ERs via varied sets of protein-ligand interactions, reflecting their differential activities, binding affinities, and specificities. We observed xenoestrogens binding to both ERs-with affinities ranging from subnanomolar to micromolar values-and acting in a subtype-dependent fashion as full agonists or partial agonists/antagonists by using different combinations of the activation functions 1 and 2 of ERα and ERβ.
Conclusions: The precise characterization of the interactions between major environmental pollutants and two of their primary biological targets provides rational guidelines for the design of safer chemicals, and will increase the accuracy and usefulness of structure-based computational methods, allowing for activity prediction of chemicals in risk assessment.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures


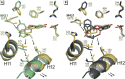
Comment in
-
EDCs and estrogen receptor activity: a pathway to safer chemical design?Environ Health Perspect. 2014 Dec;122(12):A339. doi: 10.1289/ehp.122-A339. Environ Health Perspect. 2014. PMID: 25436941 Free PMC article. No abstract available.
References
-
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. - PubMed
-
- Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93(22):12626–12630. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
