Structural libraries of protein models for multiple species to understand evolution of the renin-angiotensin system
- PMID: 25260253
- PMCID: PMC4375088
- DOI: 10.1016/j.ygcen.2014.09.010
Structural libraries of protein models for multiple species to understand evolution of the renin-angiotensin system
Abstract
The details of protein pathways at a structural level provides a bridge between genetics/molecular biology and physiology. The renin-angiotensin system is involved in many physiological pathways with informative structural details in multiple components. Few studies have been performed assessing structural knowledge across the system. This assessment allows use of bioinformatics tools to fill in missing structural voids. In this paper we detail known structures of the renin-angiotensin system and use computational approaches to estimate and model components that do not have their protein structures defined. With the subsequent large library of protein structures, we then created a species specific protein library for human, mouse, rat, bovine, zebrafish, and chicken for the system. The rat structural system allowed for rapid screening of genetic variants from 51 commonly used rat strains, identifying amino acid variants in angiotensinogen, ACE2, and AT1b that are in contact positions with other macromolecules. We believe the structural map will be of value for other researchers to understand their experimental data in the context of an environment for multiple proteins, providing pdb files of proteins for the renin-angiotensin system in six species. With detailed structural descriptions of each protein, it is easier to assess a species for use in translating human diseases with animal models. Additionally, as whole genome sequencing continues to decrease in cost, tools such as molecular modeling will gain use as an initial step in designing efficient hypothesis driven research, addressing potential functional outcomes of genetic variants with precompiled protein libraries aiding in rapid characterizations.
Keywords: Angiotensin peptides; Comparative modeling; Rat genetics; Renin-angiotensin system; Sequence-to-structure-to-function analysis.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


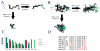



References
-
- Atanur SS, Diaz AG, Maratou K, Sarkis A, Rotival M, Game L, Tschannen MR, Kaisaki PJ, Otto GW, Ma MCJ, Keane TM, Hummel O, Saar K, Chen W, Guryev V, Gopalakrishnan K, Garrett MR, Joe B, Citterio L, Bianchi G, McBride M, Dominiczak A, Adams DJ, Serikawa T, Flicek P, Cuppen E, Hubner N, Petretto E, Gauguier D, Kwitek A, Jacob H, Aitman TJ. Genome Sequencing Reveals Loci under Artificial Selection that Underlie Disease Phenotypes in the Laboratory Rat. Cell. 2013;154:691–703. doi: 10.1016/j.cell.2013.06.040. - DOI - PMC - PubMed
-
- Batenburg WW, van den Heuvel M, van Esch J, van Veghel R, Garrelds I, Leijten F, Danser A. The (pro)renin receptor blocker handle region peptide upregulates endothelium-derived contractile factors in aliskiren-treated diabetic transgenic (mREN2)27 rats. J Hypertens Febr 2013. 2013;31:292–302. doi: 10.1097/HJH.0b013e32835c1789. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

