Novel Timothy syndrome mutation leading to increase in CACNA1C window current
- PMID: 25260352
- PMCID: PMC4907369
- DOI: 10.1016/j.hrthm.2014.09.051
Novel Timothy syndrome mutation leading to increase in CACNA1C window current
Abstract
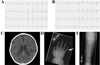
Background: Timothy syndrome (TS) is a rare multisystem genetic disorder characterized by a myriad of abnormalities, including QT prolongation, syndactyly, and neurologic symptoms. The predominant genetic causes are recurrent de novo missense mutations in exon 8/8A of the CACNA1C-encoded L-type calcium channel; however, some cases remain genetically elusive.
Objective: The purpose of this study was to identify the genetic cause of TS in a patient who did not harbor a CACNA1C mutation in exon 8/A, and was negative for all other plausible genetic substrates.
Methods: Diagnostic exome sequencing was used to identify the genetic substrate responsible for our case of TS. The identified mutation was characterized using whole-cell patch-clamp technique, and the results of these analyses were modeled using a modified Luo-Rudy dynamic model to determine the effects on the cardiac action potential.
Results: Whole exome sequencing revealed a novel CACNA1C mutation, p.Ile1166Thr, in a young male with diagnosed TS. Functional electrophysiologic analysis identified a novel mechanism of TS-mediated disease, with an overall loss of current density and a gain-of-function shift in activation, leading to an increased window current. Modeling studies of this variant predicted prolongation of the action potential as well as the development of spontaneous early afterdepolarizations.
Conclusion: Through expanded whole exome sequencing, we identified a novel genetic substrate for TS, p.Ile1166Thr-CACNA1C. Electrophysiologic experiments combined with modeling studies have identified a novel TS mechanism through increased window current. Therefore, expanded genetic testing in cases of TS to the entire CACNA1C coding region, if initial targeted testing is negative, may be warranted.
Keywords: CACNA1C; Genetics; Timothy syndrome; Whole exome sequencing; Window current.
Copyright © 2015 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The other authors have no conflicts of interest relevant to this article to disclose.
Figures


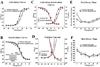

References
-
- Splawski I, Timothy KW, Sharpe LM, et al. Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. - PubMed
-
- Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R. Timothy Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews [Internet] Seattle (WA): University of Washington, Seattle; 2006. [Updated 2011]
-
- Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

