Rhodopsin management during the light-dark cycle of Anopheles gambiae mosquitoes
- PMID: 25260623
- PMCID: PMC4498666
- DOI: 10.1016/j.jinsphys.2014.09.006
Rhodopsin management during the light-dark cycle of Anopheles gambiae mosquitoes
Abstract
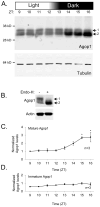
The tropical disease vector mosquito Anopheles gambiae possesses 11 rhodopsin genes. Three of these, GPROP1, GPROP3, and GPROP4, encode rhodopsins with >99% sequence identity. We created antisera against these rhodopsins and used immunohistology to show that one or more of these rhodopsins are expressed in the major R1-6 photoreceptor class of the adult A.gambiae eye. Under dark conditions, rhodopsin accumulates within the light-sensitive rhabdomere of the photoreceptor. Light treatment, however, causes extensive movement of rhodopsin to the cytoplasmic compartment. Protein electrophoresis showed that the rhodopsin is present in two different forms. The larger form is an immature species that is deglycosylated during the posttranslational maturation process to generate the smaller, mature form. The immature form is maintained at a constant level regardless of lighting conditions. These results indicate that rhodopsin biosynthesis and movement into the rhabdomere occurs at a constant rate. In contrast, the mature form increases in abundance when animals are placed in dark conditions. Light-triggered internalization and protein degradation counteracts this rhodopsin increase and keeps rhabdomeric rhodopsin levels low in light conditions. The interplay of the constant maturation rate with light-triggered degradation causes rhodopsin to accumulate within the rhabdomere only in dark conditions. Thus, Anopheles photoreceptors possess a mechanism for adjusting light sensitivity through light-dependent control of rhodopsin levels and cellular location.
Keywords: Light adaptation; Mosquito vision; Photoreceptor; Rhodopsin cycling; Visual pigment.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Light-Driven Processes Control Both Rhodopsin Maturation and Recycling in Mosquito Photoreceptors.J Neurosci. 2016 Oct 26;36(43):11051-11058. doi: 10.1523/JNEUROSCI.1754-16.2016. J Neurosci. 2016. PMID: 27798185 Free PMC article.
-
Rhodopsin coexpression in UV photoreceptors of Aedes aegypti and Anopheles gambiae mosquitoes.J Exp Biol. 2014 Mar 15;217(Pt 6):1003-8. doi: 10.1242/jeb.096347. Epub 2013 Dec 5. J Exp Biol. 2014. PMID: 24311804 Free PMC article.
-
The Drosophila rhodopsin cytoplasmic tail domain is required for maintenance of rhabdomere structure.FASEB J. 2007 Feb;21(2):449-55. doi: 10.1096/fj.06-6530com. Epub 2006 Dec 11. FASEB J. 2007. PMID: 17158966
-
Photoreceptor processes: some problems and perspectives.J Exp Zool. 1975 Oct;194(1):89-101. doi: 10.1002/jez.1401940107. J Exp Zool. 1975. PMID: 453 Review.
-
Photophobic responses and phototaxis in Chlamydomonas are triggered by a single rhodopsin photoreceptor.FEBS Lett. 1994 Mar 14;341(1):5-9. doi: 10.1016/0014-5793(94)80229-7. FEBS Lett. 1994. PMID: 8137921 Review.
Cited by
-
Multimodal synergisms in host stimuli drive landing response in malaria mosquitoes.Sci Rep. 2021 Apr 1;11(1):7379. doi: 10.1038/s41598-021-86772-4. Sci Rep. 2021. PMID: 33795798 Free PMC article.
-
Olfaction, experience and neural mechanisms underlying mosquito host preference.J Exp Biol. 2018 Feb 27;221(Pt 4):jeb157131. doi: 10.1242/jeb.157131. J Exp Biol. 2018. PMID: 29487141 Free PMC article. Review.
-
Different as night and day: Behavioural and life history responses to varied photoperiods in Daphnia magna.Mol Ecol. 2019 Oct;28(19):4422-4438. doi: 10.1111/mec.15230. Epub 2019 Sep 26. Mol Ecol. 2019. PMID: 31486145 Free PMC article.
-
Light-Driven Processes Control Both Rhodopsin Maturation and Recycling in Mosquito Photoreceptors.J Neurosci. 2016 Oct 26;36(43):11051-11058. doi: 10.1523/JNEUROSCI.1754-16.2016. J Neurosci. 2016. PMID: 27798185 Free PMC article.
-
A handmade trap for malaria mosquito surveillance by citizens in Rwanda.PLoS One. 2022 May 11;17(5):e0266714. doi: 10.1371/journal.pone.0266714. eCollection 2022. PLoS One. 2022. PMID: 35544478 Free PMC article.
References
-
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. - PubMed
-
- Benedict M, Besansky N, Chang H, Mukabayire O, Collins F. Mutations in the Anopheles gambiae pink-eye and white genes define distinct, tightly linked eye-color loci. J Hered. 1996;87:48–53.
-
- Blest A. The rapid synthesis and destruction of photoreceptor membrane by a Dinopid spider: a daily cycle. Proc R Soc Lond B. 1978;200:463–483.
-
- Blest AD. Photoreceptor membrane turnover in arthropods: comparative studies of breakdown processes and their implications. In: Williams TP, Baker BN, editors. The Effects of Constant Light on Visual Processes. Springer; US: 1980. pp. 217–245.
-
- Blest AD, Kao L, Powell K. Photoreceptor membrane breakdown in the spider Dinopis: the fate of rhabdomere products. Cell Tissue Res. 1978;195:425–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

