Heat shock protein-mediated protection against Cisplatin-induced hair cell death
- PMID: 25261194
- PMCID: PMC4310861
- DOI: 10.1007/s10162-014-0491-7
Heat shock protein-mediated protection against Cisplatin-induced hair cell death
Abstract
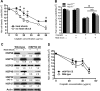
Cisplatin is a highly successful and widely used chemotherapy for the treatment of various solid malignancies in both adult and pediatric patients. Side effects of cisplatin treatment include nephrotoxicity and ototoxicity. Cisplatin ototoxicity results from damage to and death of cells in the inner ear, including sensory hair cells. We showed previously that heat shock inhibits cisplatin-induced hair cell death in whole-organ cultures of utricles from adult mice. Since heat shock protein 70 (HSP70) is the most upregulated HSP in response to heat shock, we investigated the role of HSP70 as a potential protectant against cisplatin-induced hair cell death. Our data using utricles from HSP70 (-/-) mice indicate that HSP70 is necessary for the protective effect of heat shock against cisplatin-induced hair cell death. In addition, constitutive expression of inducible HSP70 offered modest protection against cisplatin-induced hair cell death. We also examined a second heat-inducible protein, heme oxygenase-1 (HO-1, also called HSP32). HO-1 is an enzyme responsible for the catabolism of free heme. We previously showed that induction of HO-1 using cobalt protoporphyrin IX (CoPPIX) inhibits aminoglycoside-induced hair cell death. Here, we show that HO-1 also offers significant protection against cisplatin-induced hair cell death. HO-1 induction occurred primarily in resident macrophages, with no detectable expression in hair cells or supporting cells. Depletion of macrophages from utricles abolished the protective effect of HO-1 induction. Together, our data indicate that HSP induction protects against cisplatin-induced hair cell death, and they suggest that resident macrophages mediate the protective effect of HO-1 induction.
Figures



References
-
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. - PubMed
-
- Blancou P, Tardif V, Simon T, Remy S, Carreno L, Kalergis A, Anegon I. Immunoregulatory properties of heme oxygenase-1. Methods Mol Biol. 2011;677:247–268. - PubMed
-
- Caronia D, Patino-Garcia A, Milne RL, Zalacain-Diez M, Pita G, Alonso MR, Moreno LT, Sierrasesumaga-Ariznabarreta L, Benitez J, Gonzalez-Neira A. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

