mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function
- PMID: 25261477
- PMCID: PMC4201970
- DOI: 10.4049/jimmunol.1401558
mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function
Abstract
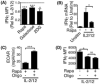
The mammalian target of rapamycin complex 1 (mTORC1) is a key regulator of cellular metabolism and also has fundamental roles in controlling immune responses. Emerging evidence suggests that these two functions of mTORC1 are integrally linked. However, little is known regarding mTORC1 function in controlling the metabolism and function of NK cells, lymphocytes that play key roles in antiviral and antitumor immunity. This study investigated the hypothesis that mTORC1-controlled metabolism underpins normal NK cell proinflammatory function. We demonstrate that mTORC1 is robustly stimulated in NK cells activated in vivo and in vitro. This mTORC1 activity is required for the production of the key NK cell effector molecules IFN-γ, which is important in delivering antimicrobial and immunoregulatory functions, and granzyme B, a critical component of NK cell cytotoxic granules. The data reveal that NK cells undergo dramatic metabolic reprogramming upon activation, upregulating rates of glucose uptake and glycolysis, and that mTORC1 activity is essential for attaining this elevated glycolytic state. Directly limiting the rate of glycolysis is sufficient to inhibit IFN-γ production and granzyme B expression. This study provides the highly novel insight that mTORC1-mediated metabolic reprogramming of NK cells is a prerequisite for the acquisition of normal effector functions.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



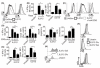

References
-
- Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, Graeber TG, Reue K, Brooks DG, Bensinger SJ. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

