Proteopathic tau seeding predicts tauopathy in vivo
- PMID: 25261551
- PMCID: PMC4205609
- DOI: 10.1073/pnas.1411649111
Proteopathic tau seeding predicts tauopathy in vivo
Abstract
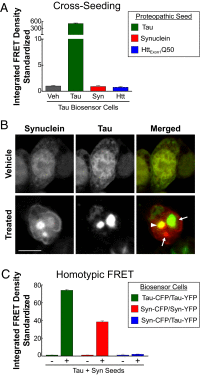
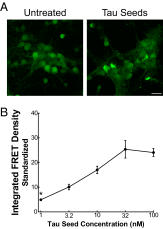
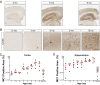
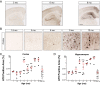
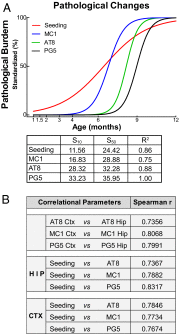
Transcellular propagation of protein aggregates, or proteopathic seeds, may drive the progression of neurodegenerative diseases in a prion-like manner. In tauopathies such as Alzheimer's disease, this model predicts that tau seeds propagate pathology through the brain via cell-cell transfer in neural networks. The critical role of tau seeding activity is untested, however. It is unknown whether seeding anticipates and correlates with subsequent development of pathology as predicted for a causal agent. One major limitation has been the lack of a robust assay to measure proteopathic seeding activity in biological specimens. We engineered an ultrasensitive, specific, and facile FRET-based flow cytometry biosensor assay based on expression of tau or synuclein fusions to CFP and YFP, and confirmed its sensitivity and specificity to tau (∼ 300 fM) and synuclein (∼ 300 pM) fibrils. This assay readily discriminates Alzheimer's disease vs. Huntington's disease and aged control brains. We then carried out a detailed time-course study in P301S tauopathy mice, comparing seeding activity versus histological markers of tau pathology, including MC1, AT8, PG5, and Thioflavin S. We detected robust seeding activity at 1.5 mo, >1 mo before the earliest histopathological stain. Proteopathic tau seeding is thus an early and robust marker of tauopathy, suggesting a proximal role for tau seeds in neurodegeneration.
Keywords: aging; amyloid; dementia; neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- R01 NS071835/NS/NINDS NIH HHS/United States
- F31 NS079039/NS/NINDS NIH HHS/United States
- S10 RR0227552/RR/NCRR NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- F32 NS087805/NS/NINDS NIH HHS/United States
- P50AG05681/AG/NIA NIH HHS/United States
- 1F32NS087805/NS/NINDS NIH HHS/United States
- 1F31NS079039/NS/NINDS NIH HHS/United States
- NS057105/NS/NINDS NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- 1R01NS071835/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

