Myocardial matrix metalloproteinase-2: inside out and upside down
- PMID: 25261607
- PMCID: PMC4312173
- DOI: 10.1016/j.yjmcc.2014.09.016
Myocardial matrix metalloproteinase-2: inside out and upside down
Abstract
Since their inaugural discovery in the early 1960s, matrix metalloproteinases (MMPs) have been shown to mediate multiple physiological and pathological processes. In addition to their canonical function in extracellular matrix (ECM) remodeling, research in the last decade has highlighted new MMP functions, including proteolysis of novel substrates beyond ECM proteins, MMP localization to subcellular organelles, and proteolysis of susceptible intracellular proteins in those subcellular compartments. This review will provide a comparison of the extracellular and intracellular roles of MMPs, illustrating that MMPs are far more interesting than the one-dimensional view originally taken. We focus on the roles of MMP-2 in cardiac injury and repair, as this is one of the most studied MMPs in the cardiovascular field. We will highlight how understanding all dimensions, such as localization of activity and timing of interventions, will increase the translational potential of research findings. Building upon old ideas and turning them inside out and upside down will help us to better understand how to move the MMP field forward.
Keywords: Cardiovascular disease; Doxycycline; Heart failure; MMP-2; Myocardial ischemia and infarction; TIPTOP.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
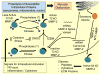
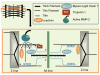
Similar articles
-
Matrix Metalloproteinases: Pathophysiologic Implications and Potential Therapeutic Targets in Cardiovascular Disease.Biomolecules. 2025 Apr 17;15(4):598. doi: 10.3390/biom15040598. Biomolecules. 2025. PMID: 40305314 Free PMC article. Review.
-
Multifunctional intracellular matrix metalloproteinases: implications in disease.FEBS J. 2021 Dec;288(24):7162-7182. doi: 10.1111/febs.15701. Epub 2021 Jan 22. FEBS J. 2021. PMID: 33405316 Review.
-
Junctophilin-2 is a target of matrix metalloproteinase-2 in myocardial ischemia-reperfusion injury.Basic Res Cardiol. 2019 Sep 10;114(6):42. doi: 10.1007/s00395-019-0749-7. Basic Res Cardiol. 2019. PMID: 31506724
-
Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects.Biochim Biophys Acta Mol Cell Res. 2017 Nov;1864(11 Pt A):1927-1939. doi: 10.1016/j.bbamcr.2017.06.009. Epub 2017 Jun 19. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28636874 Review.
-
The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease.Int J Mol Sci. 2024 Dec 21;25(24):13691. doi: 10.3390/ijms252413691. Int J Mol Sci. 2024. PMID: 39769454 Free PMC article. Review.
Cited by
-
Role of MMP-2 and CD147 in kidney fibrosis.Open Life Sci. 2022 Sep 14;17(1):1182-1190. doi: 10.1515/biol-2022-0482. eCollection 2022. Open Life Sci. 2022. PMID: 36185410 Free PMC article. Review.
-
Increased Circulating Tissue Inhibitor of Metalloproteinase-2 Is Associated With Resistant Hypertension.J Clin Hypertens (Greenwich). 2016 Oct;18(10):969-975. doi: 10.1111/jch.12865. Epub 2016 Jul 14. J Clin Hypertens (Greenwich). 2016. PMID: 27412873 Free PMC article.
-
The protective role of GATA6+ pericardial macrophages in pericardial inflammation.iScience. 2024 Jun 13;27(7):110244. doi: 10.1016/j.isci.2024.110244. eCollection 2024 Jul 19. iScience. 2024. PMID: 39040070 Free PMC article.
-
Intracellular Localization in Zebrafish Muscle and Conserved Sequence Features Suggest Roles for Gelatinase A Moonlighting in Sarcomere Maintenance.Biomedicines. 2019 Nov 29;7(4):93. doi: 10.3390/biomedicines7040093. Biomedicines. 2019. PMID: 31795436 Free PMC article.
-
MMP-2 Associates With Incident Heart Failure and Atrial Fibrillation: The ARIC Study.Circ Heart Fail. 2023 Nov;16(11):e010849. doi: 10.1161/CIRCHEARTFAILURE.123.010849. Epub 2023 Sep 27. Circ Heart Fail. 2023. PMID: 37753653 Free PMC article.
References
-
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–78. - PubMed
-
- Jacob-Ferreira AL, Schulz R. Activation of intracellular matrix metalloproteinase-2 by reactive oxygen-nitrogen species: Consequences and therapeutic strategies in the heart. Arch Biochem Biophys. 2013;540:82–93. - PubMed
-
- Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol. 2013;87:1–11. - PubMed
-
- Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HV-00244/HV/NHLBI NIH HHS/United States
- R24 DK092154/DK/NIDDK NIH HHS/United States
- R01HL075360/HL/NHLBI NIH HHS/United States
- R21 AT004277/AT/NCCIH NIH HHS/United States
- T32HL105324/HL/NHLBI NIH HHS/United States
- T32 HL105324/HL/NHLBI NIH HHS/United States
- MOP77526/CAPMC/ CIHR/Canada
- AT4277/AT/NCCIH NIH HHS/United States
- R01 HL043617/HL/NHLBI NIH HHS/United States
- MOP66953/CAPMC/ CIHR/Canada
- HHSN268201000036C/HL/NHLBI NIH HHS/United States
- I01 BX000505/BX/BLRD VA/United States
- R01 HL075360/HL/NHLBI NIH HHS/United States
- DK92154/DK/NIDDK NIH HHS/United States
- HHSN 268201000036C/PHS HHS/United States
- HL43617/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

